Researchers identify deletions in long noncoding RNA that lead to severe neurodevelopmental disorder
Powered by WPeMatico
Powered by WPeMatico

New Delhi: In an effort to ensure access to affordable and quality medicines, Prime Minister Narendra Modi is set to inaugurate a new Jan Aushadhi Kendra at the All India Institute of Medical Sciences (AIIMS), New Delhi, on October 29.
The event will also be attended by Jagat Prakash Nadda, the Union Minister of Chemicals and Fertilizers and Health and Family Welfare.
The main objective of this Kendra will be to provide affordable and quality medicines to the patients visiting AIIMS for the treatment of various ailments.
According to an ANI report, the press release stated that the new Kendra, spanning an area of 1,724 square feet, aims to provide over 2,047 quality generic medicines and 300 surgical devices at prices significantly lower than their branded counterparts.
Also Read:Dr Mansukh Mandaviya inaugurates Credit Assistance Program for Jan Aushadhi Kendras
The available medicines will cover a wide array of therapeutic groups, including cardiovascular health, anti-cancer treatments, anti-diabetics, anti-infectives, anti-allergic medications, gastrointestinal solutions, and nutraceuticals.
This initiative will revolutionize healthcare access for thousands of patients each day who flock to AIIMS, New Delhi each day in search of affordable, high-quality medical solutions.
The establishment of this Jan Aushadhi Kendra represents a monumental leap forward in the government’s commitment to ensuring that essential medicines are not just a privilege for the few, but a right for all.
In a bustling medical hub like AIIMS, renowned for its cutting-edge treatments and expert care, this Kendra will serve as a beacon of hope and illuminate the path toward equitable healthcare for all citizens, regardless of their economic background.
Presently, more than 14,000 Jan Aushadhi Kendras are operating across India, delivering health benefits to approximately one million people every single day.
These Kendras extend their life-saving services into the farthest corners of the nation, reaching the most remote and underserved areas, across 780 districts.
The Government of India is not resting on its laurels; ambitious plans are underway to expand this formidable network to 25,000 Kendras over the next two years.
This visionary expansion will not only enhance the availability of affordable healthcare but will also empower millions of citizens to access the medicines they need, fostering a healthier, more resilient India for generations to come.
The inauguration of Jan Aushadhi Kendra at AIIMS marks a historic milestone in the relentless efforts of the Pradhan Mantri Bhartiya Janaushadhi Pariyojana (PMBJP) to reshape the healthcare landscape of India, news agency ANI reported.
This Kendra is not merely an addition to the existing facilities; it stands as a beacon of hope for countless patients seeking affordable treatment options in New Delhi.
By providing a vast array of high-quality generic medicines at prices that are a fraction of branded counterparts, this initiative will serve as an indispensable lifeline for those who have long struggled with the prohibitive costs of healthcare.
It symbolizes a transformative shift towards a more inclusive healthcare system, where quality treatment is accessible to all, regardless of socioeconomic status.
This initiative also reaffirms the government’s steadfast dedication to promoting health equity, ensuring that no citizen is left behind in the pursuit of quality healthcare.
It embodies the vision of a fairer society where every individual, from urban centres to remote villages, can access essential medicines without financial strain.
The Jan Aushadhi Kendra at AIIMS is a powerful testament to the government’s commitment to fostering a healthier nation, empowering individuals with the right to affordable healthcare, and fundamentally transforming the way healthcare is perceived and delivered across India. With this Kendra, we are not just improving health outcomes; we are igniting hope and restoring dignity to countless lives.
Also Read:Pradhan Mantri Bhartiya Janaushadhi Pariyojana achieves target of Rs 1000 crore in sales in FY 2023-24
Powered by WPeMatico
USA: Recent
research indicates that sacubitril/valsartan can be safely continued in heart
failure patients, maintaining cardiovascular and kidney benefits even as eGFR
drops below 30 mL/min/1.73 m².
The study
published in JACC: Heart Failure highlights sacubitril/valsartan’s
safety despite worsening kidney function, challenging the current clinical guidelines
to stop ARNI (angiotensin receptor-neprilysin inhibitor) below eGFR 30
mL/min/1.73 m², and also emphasizes the need for close monitoring of such
patients and further research.
Heart
failure (HF) and chronic kidney disease (CKD) are significant causes of
morbidity and mortality, frequently coexisting, with CKD affecting nearly 50%
of HF patients. Sacubitril/valsartan, an angiotensin receptor-neprilysin
inhibitor (ARNI), is recommended by guidelines for managing heart failure with
reduced ejection fraction (HFrEF), with its benefits also extending to patients
with mildly reduced or preserved ejection fraction, especially those with an EF
below normal (<60%).
The U.S.
Food and Drug Administration (FDA) recommends sacubitril/valsartan for the
treatment of heart failure. While the FDA does not specify a renal function
threshold that would prevent its initiation or continuation, it advises
starting the drug at the lowest dose (24/26 mg twice daily) for patients with
an estimated glomerular filtration rate (eGFR) below 30 mL/min/1.73 m².
The
PARADIGM-HF and PARAGON-HF trials demonstrated consistent benefits of
sacubitril/valsartan relative to RAS inhibitors in patients with eGFR as low as
25-30 mL/min/1.73 m² but lacked data on the safety and efficacy of
sacubitril/valsartan in heart failure patients experiencing further eGFR
decline below inclusion thresholds.
This
prompted Safia Chatur, MD, Cardiovascular Division, Brigham and Women’s
Hospital, Harvard Medical School, Boston, Massachusetts, USA, and colleagues to
assess the safety and efficacy of continuing sacubitril/valsartan in patients
with deterioration of kidney function below an eGFR of 30mL/min/1.73 m2.
For the
study, researchers evaluated the association between a deterioration in eGFR using
time-updated Cox models in post hoc parallel trial analyses of PARADIGM-HF and PARAGON-HF.
The findings of the study were as follows:
The researchers concluded that while the balance of efficacy and safety supports the continued use of sacubitril/valsartan for cardiovascular and kidney protection, close monitoring of these patients is essential. They also emphasized the need for randomized studies in heart failure populations with advanced chronic kidney disease.
Reference: Chatur, S., Beldhuis, I. E., Claggett, B.
L., McCausland, F. R., Neuen, B. L., Desai, A. S., Rouleau, J. L., Zile, M. R.,
Packer, M., Pfeffer, M. A., Lefkowitz, M. P., McMurray, J. J. V., Solomon, S.
D., & Vaduganathan, M. (2024). Sacubitril/valsartan in patients with heart
failure and deterioration in eGFR to <30 mL/min/1.73 m². JACC: Heart
Failure, 12(10), 1704-1706. https://doi.org/10.1016/j.jchf.2024.03.014
Powered by WPeMatico

Himachal Pradesh: The state government has been actively working to integrate palliative care into the healthcare system of Himachal Pradesh which is a specialized medical care that aims at providing relief from pain, discomfort and stress to patients suffering from other serious ailments. This was stated by Health Minister Dr. (Col.) Dhani Ram Shandil who is leading a senior delegation, to study Pallium India’s palliative care model involving community in Kerala from 23rd to 26th October 2024.
He said that the purpose of the visit was to explore palliative care, its potential and scope of its implementation in Himachal Pradesh through the state healthcare delivery system.
The visit aims to develop a customized palliative care framework for Himachal and integrate similar practices into its healthcare system, he said. The delegation also visited a palliative care hospital in Trivandrum and made home visits to witness the practice, procedure and effectiveness of palliative care delivery. The Health Minister would also meet former Chief Secretary of Kerala S M Vijayanand engaged in policy discussions regarding palliative care.
Read also: Himachal Pradesh health institutions to be upgraded soon: CM Sukhvinder Singh Sukhu
He added that Pallium India and the National Health Mission (NHM) Himachal Pradesh signed a Memorandum of Understanding (MoU) in February, 2024 to further strengthen palliative care centres in the state. As per the MoU, Pallium India would provide palliative care training to medical officers, community Health officers, Health workers, Asha workers and other medical staff.
The delegation includes Secretary Health M. Sudha Devi, Mission Director NHM Priyanka Verma, Director Medical Education and Research Dr Rakesh Sharma, Deputy Mission Director Dr Gopal Beri and State Program Officer (NCD) Dr Anadi.
Powered by WPeMatico
A recent study suggested that metabolic-associated steatohepatitis
can be managed by a multifaceted approach that includes diabetes-related
lifestyle modifications, weight loss, and pharmacological management. The study
was published in the journal Diabetes Research and Clinical Practice.
Nonalcoholic fatty liver disease (NAFLD) renamed and
redefined as metabolic dysfunction-associated steatotic liver disease (MASLD) is
one of the most common non-communicable and global chronic liver diseases. Metabolic
dysfunction-associated steatohepatitis (MASH) is the second leading cause of end-stage
liver disease occurring due to metabolic imbalances and insulin resistance. There
is limited data on the awareness and the potential prognostic implications of
MASLD and MASH. Hence, researchers described the multifactorial pathways, the
potential complications, and the various management methods in a detailed
review.
Risk factors:
Prevalence:
Studies have shown that diabetics have an increased
predisposition to develop advanced fibrosis and MASH.
Progression:
Therapeutic management:
Thus, the study concluded that MASH is a complex condition
driven by visceral fat expansion, altered gut microbiota, insulin resistance, increased
liver fat, and stress. Lifestyle changes, weight loss, and pharmacology are the
multifaceted ways of managing MASH. However, large-scale trials are needed to
assess the long-term efficacy.
Further reading: Gancheva S, Roden M, Castera L. Diabetes as a risk factor for MASH progression. Diabetes Res Clin Pract. Published online September 6, 2024. doi:10.1016/j.diabres.2024.111846
Powered by WPeMatico
A recent observational analysis compared the choice of
surgical techniques like off-pump (OPCABG) and on-pump (ONCABG) coronary artery
bypass grafts and found that both OPCABG and ONCABG procedures are safe to
perform and have a good prognosis as per the results published in the journal
Annals of Medicine and Surgery.
Coronary artery bypass grafting (CABG) is the most common
cardiac surgery procedure despite several percutaneous procedures. Due to their
pros and cons, there has been long ambiguity on the efficiency of on-pump
(ONCABG) and off-pump (OPCABG) CABG methods. OPCABG is complex requires a
skilled surgeon and compromises the technical performance of the surgeon, while
ONCABG requires a heart-lung machine. Hence, researchers from the University
Hospital and the University of Turku, Finland conducted a study to determine which
surgical technique affects prognosis in coronary artery bypass grafting (CABG).
They performed a single-center, retrospective observational
study on all the off-pump (OPCABG) and on-pump (ONCABG) patients at Turku
University Central Hospital. Patients who underwent concomitant valve surgery
and re-operative CABG were excluded. The main outcome of measurement was the length
of stay in the intensive care unit (ICU), need for resternotomy, perioperative
mortality, and 1-year mortality.
Statistical analyses were performed with R 4.0.2 software. Numerical
variables between ONCABG and OPCABG groups were compared using the Mann-Whitney
U test. One-to-one propensity score matching was used to account for bias in
patient selection.
Findings:
Thus, the study concluded that surgical outcomes were
equally good between the two groups at 1-year and 3-year follow-ups. Despite low-risk
profiles and preoperative characteristics, the ONCABG patients received more
often red blood cells and higher amounts of crystalloids and had higher
postoperative TnT and lactate values. They also suggested that the OPCABG
technique may be particularly suitable for frail patients due to milder myocardial
damage and more balanced postoperative hemodynamics.
Further reading: Rantanen M, Yousif R, Kallioinen M, et al. Retrospective observational analysis of a coronary artery bypass grafting surgery patient cohort: Off-pump versus on-pump. Ann Med Surg (Lond). 2022;84:104812. Published 2022 Nov 9. doi:10.1016/j.amsu.2022.104812
Powered by WPeMatico
A new study published in the Journal of American Medical Association found that children and adolescents aged 10 to 19 who were diagnosed with COVID-19 had a higher chance of an incident diagnosis of type 2 diabetes than the ones who were diagnosed with other respiratory diseases. The patients who are infected with SARS-CoV-2 are at risk for developing diabetes and a host of other chronic illnesses. The Centres for Disease Control and Prevention (CDC) recorded an increased incidence of diabetes among patients under the age of 18 following COVID-19 based on health claims data, but, did not differentiate between type 1 (T1D) and type 2 (T2D) diabetes, even though the majority of research involved adults. Margaret Miller and the team carried out this investigation to look out if the likelihood of incident T2D diagnosis in children is elevated in the 6 months following SARS-CoV-2 infection.
The electronic health records obtained through TriNetX analytics platforms from January 1, 2020, to December 31, 2022, were utilized in this retrospective cohort research. Children without diabetes who were between the ages of 10 and 19 were included. Data analysis was carried out from August 15 to September 15, 2023, with further studies conducted in January 20 and August 8 to 13, 2024. The primary exposure was a diagnosis of COVID-19 or a respiratory illness that was not COVID-19. The main result was the comparison of new T2D diagnoses at 1, 3, and 6 months following index infection by risk ratios (RRs) and 95% confidence intervals (CIs).
After propensity score matching, a total of 3,06,801 patients with COVID-19 and 3,06,801 patients with other respiratory infections (ORIs) but no reported COVID-19 were included in the primary research group, which comprised 6,13,602 individuals. When compared to the matched group with ORIs, the risk of receiving a new diagnosis of T2D was substantially higher from the day of infection to 1, 3, and 6 months following COVID-19 diagnosis. The hospitalized group and the subpopulation categorized as overweight or obese had similar outcomes. Overall, the pediatric patients aged 10 to 19 years had a higher likelihood of a new T2D diagnosis following COVID-19 infection than other children with ORIs.
Source:
Miller, M. G., Terebuh, P., Kaelber, D. C., Xu, R., & Davis, P. B. (2024). SARS-CoV-2 Infection and New-Onset Type 2 Diabetes Among Pediatric Patients, 2020 to 2022. In JAMA Network Open (Vol. 7, Issue 10, p. e2439444). American Medical Association (AMA). https://doi.org/10.1001/jamanetworkopen.2024.39444
Powered by WPeMatico

A new study published in the British Journal of Nutrition showed that daily consumption of 2 ounces of raw almonds in a self-selected diet for 16 weeks were well tolerated hedonistically and enhanced diet quality without encouraging weight gain in people with increased HbA1c concentrations.
There are three primary forms of diabetes mellitus as;
Consuming almonds may help reduce post-prandial glycemia, according to mounting data. However, they have varying effects on HbA1c, a measure of long-term glycemic management. This study was to investigate if long-term almond intake can lower HbA1c levels in the individuals with increased HbA1c levels.
This parallel-arm, randomized, controlled study lasted 16 weeks where 81 people with high HbA1c levels (>5.7%) were randomized to include either energy-matched snacks (pretzels, C group; N=42) or two servings (2 oz) of raw almonds (A group; N=39) in their daily diets. The goal was to consume half of these intervention items for breakfast and the other half as a midmorning or midafternoon snack substitute. Body weight, plasma lipids, body composition, HbA1c, alpha and gamma-tocopherol, glycemia (as determined by a meal tolerance test), continuous glucose monitoring, dietary intake, and hedonic reactions to test meals were all measured during the intervention period.
Although the individuals who ate almonds consumed 253 kcal/d more than the ones in the control group (p=0.02), there was no discernible difference in body weight. Blood chemistry, body composition, glycemia, and HbA1c concentrations did not differ statistically significantly over time or across groups. In contrast to the control group, the Healthy Eating Index ratings of the almond group increased.
Furthermore, the almond group’s hedonic assessment of almonds did not decrease when compared to the decreased preference for the pretzel snack of control group. In the almond group, alpha-tocopherol increased noticeably, whereas gamma-tocopherol mostly tended to fall by suggesting adherence to the dietary intervention. Overall, adding 2 ounces of raw almonds to the regular diet of people with high HbA1c concentrations did not lower HbA1c levels or enhance cardiovascular or glycemic indices over the short or medium term.
Source:
Huang, L.-C., Henderson, G. C., & Mattes, R. D. (2024). Effects of Daily Almond Consumption on Glycemia in Adults with Elevated Risk for Diabetes: A Randomized Controlled Trial. In British Journal of Nutrition (pp. 1–35). Cambridge University Press (CUP). https://doi.org/10.1017/s0007114524001053
Powered by WPeMatico
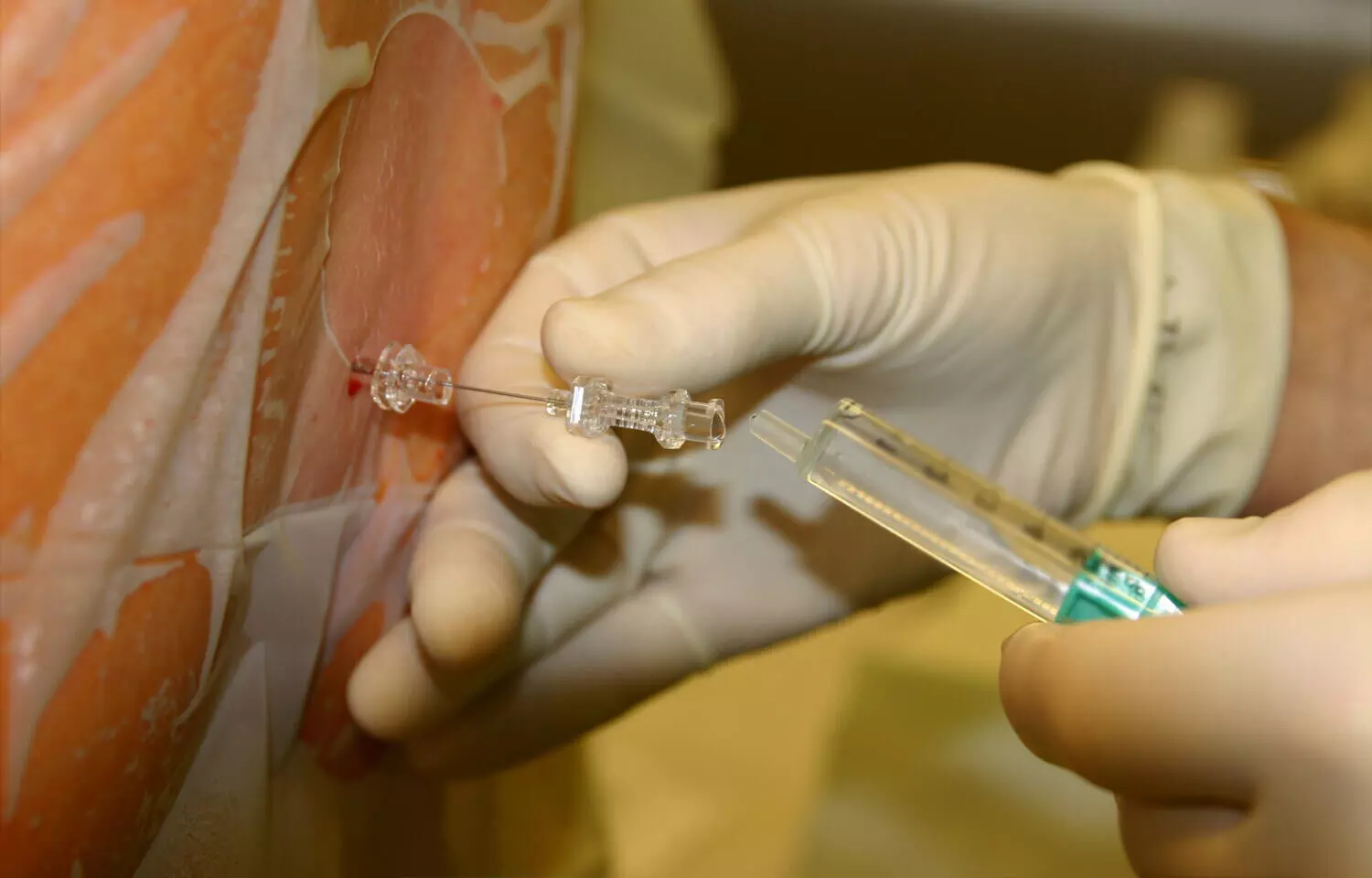
Recent study explored the use of thoracic spinal anesthesia (TSA) as an alternative to general anesthesia (GA) for short-duration breast surgeries. The researchers conducted a randomized, non-blinded study comparing the two anesthetic approaches on factors such as time to first rescue analgesic, patient satisfaction, recovery time, and cost. The study included 30 patients scheduled for elective breast surgeries less than 90 minutes long. Group I received TSA while Group II received standard GA. The primary outcome was time to first rescue analgesic, and secondary outcomes were time to recovery, patient satisfaction, and cost.
The results showed the time to first rescue analgesic was significantly longer in the TSA group compared to the GA group (336 vs. 188 minutes, p=0.001). Patient satisfaction scores were also superior in the TSA group (p=0.002). Recovery was quicker in the TSA group as evidenced by better Aldrete scores (p=0.001). The average cost was lower in the TSA group compared to the GA group (p=0.002). There were no significant hemodynamic disturbances or major complications in either group.
Conclusion
The researchers concluded that TSA is an excellent alternative to GA for short-duration breast surgeries in terms of analgesic efficacy, patient satisfaction, recovery, and cost-effectiveness. TSA provided better pain control, faster recovery, and higher patient satisfaction compared to GA, without major adverse effects. The researchers suggest TSA should be considered as a safe and effective anesthetic technique for appropriate breast surgery cases.
Key Points
1. The study explored the use of thoracic spinal anesthesia (TSA) as an alternative to general anesthesia (GA) for short-duration breast surgeries.
2. The study was a randomized, non-blinded trial that compared TSA (Group I) to standard GA (Group II) in 30 patients undergoing elective breast surgeries less than 90 minutes long.
3. The primary outcome was time to first rescue analgesic, and secondary outcomes were time to recovery, patient satisfaction, and cost.
4. The results showed the time to first rescue analgesic was significantly longer in the TSA group compared to the GA group (336 vs. 188 minutes, p=0.001). Patient satisfaction scores were also superior in the TSA group (p=0.002). Recovery was quicker in the TSA group as evidenced by better Aldrete scores (p=0.001). The average cost was lower in the TSA group compared to the GA group (p=0.002).
5. There were no significant hemodynamic disturbances or major complications in either group.
6. The researchers concluded that TSA is an excellent alternative to GA for short-duration breast surgeries in terms of analgesic efficacy, patient satisfaction, recovery, and cost-effectiveness, and should be considered a safe and effective anesthetic technique for appropriate breast surgery cases.
Reference –K a r t h i k G S , S r i n i v a s a n R , Sudheer R, Amabareesha M, Monisha TS, Kumar MD. Thoracicspinal anaesthesia – An effective alternative to general anaesthesia in breast surgeries: A randomised, non‑blinded study. Indian J Anaesth 2024;68:902‑8.
Powered by WPeMatico

A new, computerized, mental health assessment tool may allow doctors to quickly identify children experiencing anxiety or depression before surgery, suggests new research presented at the ANESTHESIOLOGY® 2024 annual meeting. In the small, single-center study, researchers found more than half of the children screened had anxiety before having surgery and more than one-third had depression.
“The use of the KCAT® tool in pediatric patients in the preoperative setting is very feasible and the results of our pilot study show a substantial prevalence of these mental health conditions in this surgical population,” said Elizabeth Pealy, M.D., lead author of the study and assistant professor of anesthesia and critical care at the University of Chicago Medicine. “Anxiety and depression are caused by many factors. Many kids are anxious before having surgery and the stress of undergoing the actual procedure can accentuate it.”
The overall incidence and prevalence of anxiety and depression in children in the United States has dramatically increased in recent years. It is difficult for pediatric anesthesiologists to determine if patients have undiagnosed anxiety or depression, or severe preoperative anxiety, prior to the surgery. Increased anxiety can contribute to the child being uncooperative during the anesthetic induction, as well as prolonged recovery, increased postoperative pain and delirium, and decreased patient satisfaction. With thousands of children undergoing surgery every year, having a comprehensive yet quick method to screen for these conditions is needed.
In the study, researchers tested the feasibility of administering KCAT-a computerized adaptive mental health assessment tool — on pediatric surgical patients in the preoperative holding area and examined the prevalence of anxiety and depression in this group. According to the authors, KCAT allows for quick and accurate assessment of anxiety and depression without the need for a trained clinician interviewer. It is administered on a tablet device and allows for real-time assessment in the perioperative setting.
Sixty-five children scheduled for elective surgery at a large children’s hospital were enrolled. The median age was 13 years old (range 7-18 years). All of the children completed the assessment (taking an average of two minutes and 13 seconds), with 15 receiving parental assistance. The researchers found 57% of patients screened positive for anxiety and 34% of patients screened positive for depression.
“This is the first study to examine the use of this tool on children in this setting,” said Sarah Nizamuddin, M.D., co-author of the study and associate professor of anesthesia and critical care at the University of Chicago Medicine. “The ability to rapidly assess for and capture anxiety and depression levels can allow providers to offer a variety of anxiety-reducing options prior to and after surgery to the patients who would benefit the most. Further studies should aim to determine how to use the information to better cater to the needs of pediatric patients to improve their experience during surgery.”
Reference:
Easy-to-use tool helps screen for anxiety, depression in children having surgery, American Society of Anesthesiologists, Meeting: Anesthesiology 2024
Powered by WPeMatico
