Surgery to treat chronic sinus disease more effective than antibiotics, clinical trial finds
Powered by WPeMatico
Powered by WPeMatico
Powered by WPeMatico
Powered by WPeMatico
Powered by WPeMatico

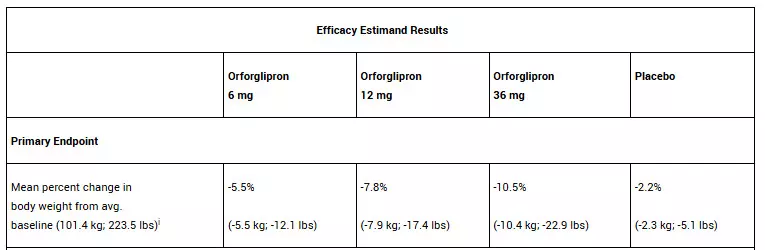
Topline results from the ATTAIN-2 trial have demonstrated that adults taking 36 mg orforglipron lost an average of 10.5% body weight at 72 weeks, with over half of participants on any dose achieving an HbA1c of 6.5% or lower. The oral GLP-1 demonstrated significant benefits for adults with obesity and type 2 diabetes.
In the trial, all three doses of orforglipron met the primary and all key secondary endpoints, delivering significant weight loss, meaningful A1C reductions, and improvements in cardiometabolic risk factors at 72 weeks. For the primary endpoint, orforglipron 36 mg, taken once per day without food and water restrictions, lowered weight by an average of 10.5% (22.9 lbs) compared to 2.2% (5.1 lbs) with placebo using the efficacy estimand.1 With the completion of ATTAIN-2, Lilly now has the full clinical data package required to initiate global regulatory submissions for orforglipron.
“Based on my experience leading clinical trials in obesity and diabetes, these data show the potential for orforglipron to offer an efficacy, safety, and tolerability profile consistent with the injectable GLP-1 class,” said Louis J. Aronne, MD, FACP, DABOM, founder and Chair Emeritus of the American Board of Obesity Medicine, former president of The Obesity Society, Fellow of the American College of Physicians, and world-renowned obesity specialist. “Orforglipron could help health care providers expand treatment options for patients who prefer oral therapies without compromising clinical results.”
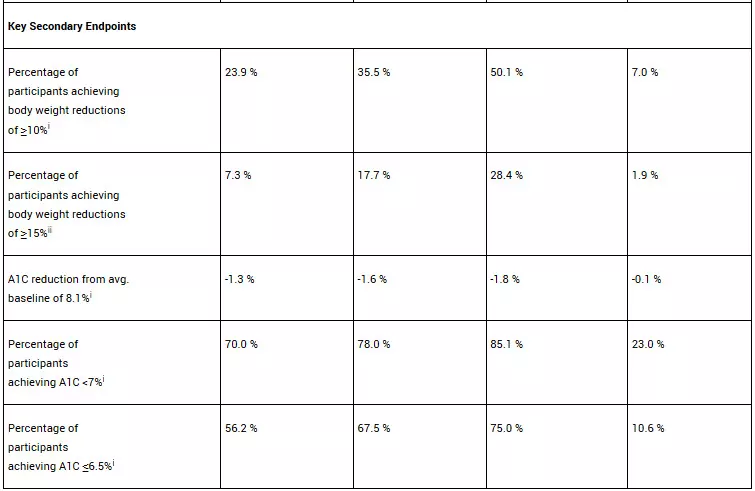
In the ATTAIN-2 trial, orforglipron met the primary endpoint of superior body weight reduction compared to placebo. Participants taking the highest dose of orforglipron lost an average of 22.9 lbs (10.5%) at 72 weeks using the efficacy estimand. In a key secondary endpoint, orforglipron lowered A1C by 1.3% to 1.8% from a baseline of 8.1% across doses. In another key secondary endpoint, 75% of participants taking the highest dose of orforglipron achieved an A1C ≤6.5%, which is at or below the American Diabetes Association’s definition of diabetes. Additionally, orforglipron showed clinically meaningful benefits across key cardiovascular risk factors, including non-HDL cholesterol, systolic blood pressure and triglycerides. In a pre-specified exploratory analysis, the highest dose of orforglipron reduced high-sensitivity C-reactive protein (hsCRP) levels, a marker of inflammation, by 50.6%.
iSuperiority test was adjusted for multiplicity with all three doses.
iiSuperiority test was adjusted for multiplicity with the 12 mg and 36 mg doses.
For the treatment-regimen estimand, each dose of orforglipron led to statistically significant improvements across the primary and all key secondary endpoints, including:
• Percent weight reduction: -5.1% (-5.3 kg; -11.7 lbs; 6 mg), -7.0% (-7.2 kg; -15.9 lbs; 12 mg), -9.6% (-9.6 kg; -21.2 lbs; 36 mg), -2.5% (-2.7 kg; -6.0 lbs; placebo)
• Percentage of participants achieving body weight reductions of ≥10%: 22.6% (6 mg), 31.2% (12 mg), 45.6% (36 mg), 9.0% (placebo)
• Percentage of participants achieving body weight reductions of ≥15%: 6.8% (6 mg), 14.4% (12 mg), 26.0% (36 mg), 3.0% (placebo)
• A1C reduction: -1.2% (6 mg), -1.5% (12 mg), -1.7% (36 mg), -0.5% (placebo)
• Percentage of participants achieving A1C <7%: 64.6% (6 mg), 75.9% (12 mg), 75.5% (36 mg), 30.5% (placebo)
• Percentage of participants achieving A1C ≤6.5%: 52.5% (6 mg), 57.6% (12 mg), 66.6% (36 mg), 15.4% (placebo)
“The ATTAIN-2 results reinforce the potential for orforglipron, as a once-daily oral, to deliver meaningful weight loss and A1C reduction, consistent with similar landmark trials for injectable GLP-1s,” said Kenneth Custer, Ph.D., Lilly executive vice president and president of Lilly Cardiometabolic Health. “With these positive data in hand, we are moving with urgency toward global regulatory submissions to potentially meet the needs of patients who are waiting. If approved, we are ready to offer a convenient, once-daily pill that can be scaled globally-removing barriers and redefining how obesity is treated around the world.”
The overall safety profile of orforglipron in ATTAIN-2 was consistent with the established GLP-1 receptor agonist class. The most commonly reported adverse events were gastrointestinal-related and generally mild-to-moderate in severity. The most common adverse events for participants treated with orforglipron (6 mg, 12 mg and 36 mg, respectively) were nausea (20.1%, 31.1% and 36.4%) vs. 8.4% with placebo, vomiting (12.8%, 20.2% and 23.1%) vs. 3.8% with placebo, diarrhea (21.3%, 24.8% and 27.4%) vs. 15.0% with placebo, constipation (17.7%, 21.1% and 22.4%) vs. 7.8% with placebo, and dyspepsia (9.1%, 15.4% and 10.9%) vs. 3.5% with placebo. Treatment discontinuation rates due to adverse events were 6.1% (6 mg), 10.6% (12 mg) and 10.6% (36 mg) for orforglipron vs. 4.6% with placebo. Overall treatment discontinuation rates were balanced across the treatment groups with 19.1% (6 mg), 22.3% (12 mg) and 20.5% (36 mg) for orforglipron vs. 20.0% with placebo. No hepatic safety signal was observed.
Detailed ATTAIN-2 results will be presented at a future medical meeting and published in a peer-reviewed journal.
About orforglipron
Orforglipron (or-for-GLIP-ron) is an investigational, once-daily small molecule (non-peptide) oral glucagon-like peptide-1 receptor agonist that can be taken any time of the day without restrictions on food and water intake.5 Orforglipron was discovered by Chugai Pharmaceutical Co., Ltd. and licensed by Lilly in 2018. Chugai and Lilly published the preclinical pharmacology data of this molecule together. Lilly is running Phase 3 studies on orforglipron for the treatment of type 2 diabetes and for weight management in adults with obesity or overweight with at least one weight-related medical problem. It is also being studied as a potential treatment for obstructive sleep apnea and hypertension in adults with obesity.
About ATTAIN-2 and ATTAIN clinical trial program
ATTAIN-2 (NCT05872620) is a Phase 3, 72-week, randomized, double-blind, placebo-controlled trial comparing the efficacy and safety of orforglipron 6 mg, 12 mg and 36 mg as monotherapy with placebo in adults with obesity or overweight and type 2 diabetes. The trial randomized over 1,600 participants across the U.S., Argentina, Australia, Brazil, China, Czechia, Germany, Greece, India, South Korea and Puerto Rico in a 1:1:1:2 ratio to receive either 6 mg, 12 mg or 36 mg orforglipron or placebo. The primary objective of the study was to demonstrate that orforglipron (6 mg, 12 mg, 36 mg) is superior to placebo in mean body weight change from baseline at 72 weeks in people with a BMI ≥27.0 kg/m² and type 2 diabetes who are on stable treatment with either diet/exercise alone or up to three oral antihyperglycemic medications. All participants receiving orforglipron started the study at a dose of 1 mg once-daily and then increased the dose in a step-wise approach at four-week intervals to their final randomized maintenance dose of 6 mg (via steps at 1 mg and 3 mg), 12 mg (via steps at 1 mg, 3 mg and 6 mg) or 36 mg (via steps at 1 mg, 3 mg, 6 mg, 12 mg and 24 mg). Dose reduction was only allowed for GI tolerability if other mitigations failed.
The ATTAIN Phase 3 global clinical development program for orforglipron has enrolled more than 4,500 people with obesity or overweight across two global registration trials.
Powered by WPeMatico

The American College of Cardiology (ACC) has released new vaccination recommendations for adults with cardiovascular disease, advising immunization against COVID-19, influenza, RSV, pneumonia, and shingles. Published in JACC, the guidelines provide evidence-based support for each vaccine and include FAQs to guide discussions between patients and clinicians as part of routine prevention and treatment.
The guidance also provides detailed evidence for each vaccine recommendation and answers to frequently asked questions to guide conversations between clinicians and patients.
“Vaccination against communicable respiratory diseases and other serious diseases is critical for people with heart disease, but barriers exist to ensuring people are educated on which vaccines to get, how often to get them and why they are important,” said Paul Heidenreich, MD, FACC, chair of the CCG writing committee. “With this document, we want to encourage clinicians to have these conversations and help their patients manage vaccination as part of a standard prevention and treatment plan.”
People with heart disease have a higher risk of infection when exposed to a respiratory virus and a higher risk of adverse outcomes, including hospitalization and death. Studies have shown that vaccines are highly effective in reducing these risks; however, a recent study showed only 30% of primary care physicians are assessing their patients’ vaccination status at clinic visits.
The ACC issued this CCG to consolidate vaccine-specific recommendations made by ACC/American Heart Association guidelines and the Centers for Disease Control and Prevention. The guidance mainly focuses on respiratory vaccines but also offers guidance based on emerging evidence that other vaccines-such as the herpes zoster (shingles) vaccine-may offer cardiovascular protective benefits.
Vaccine specific guidance includes:
• Influenza – An annual flu vaccine is recommended for all adults to reduce cardiovascular morbidity, cardiovascular mortality and all-cause death. Nasal versions of the vaccine are not recommended in patients over 50.
• Pneumococcal – recommended for adults 19 or older with heart disease to get this one-time vaccine to protect against pneumonia, bacteremia and meningitis and related risk of hospitalization and death. Following CDC/Advisory Committee on Immunization Practices recommendation, the guidance advises a single dose of PCV20 or PCV21, or PCV15 followed by PPSV23 depending on prior vaccination history.
• COVID-19 – For the 2024–25 season, all adults with heart disease were recommended to receive the seasonal COVID-19 vaccine. Future vaccination frequency may change, but it is likely vaccination will remain beneficial for those with heart disease. Benefits include reduced risk of infection, severe infection, death, heart attack, COVID-19 induced pericarditis/myocarditis, COVID-19 induced stroke and atrial fibrillation, and long COVID symptoms.
• RSV – recommended for adults 75 or older and for adults aged 50–74 with heart disease to protect against lower respiratory disease that can result in hospitalization and death. Current guidance recommends a single dose, rather than annual vaccination.
• Shingles – recommended for adults 50 or older to receive two doses to protect against increased risk of stroke and heart attack when infected. People with heart disease are at an increased risk of shingles infection.
The document outlines strategies to improve vaccination rates, address hesitancy and overcome barriers to access, noting that clinician-patient discussions about vaccination during cardiology visits can be an important opportunity to integrate vaccination into a cardiovascular care plan.
Reference:
A. Heidenreich, P, Bhatt, A, Nazir, N. et al. 2025 Concise Clinical Guidance: An ACC Expert Consensus Statement on Adult Immunizations as Part of Cardiovascular Care: A Report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol. Published online Aug. 26, 2025. doi:10.1016/j.jacc.2025.07.003.
Powered by WPeMatico
A new study published in the Journal of American Heart Association showed that degenerative valvular heart disease has been linked to an increased risk of rheumatoid arthritis (RA).
Degenerative valvular heart disease (VHD) is a prevalent subtype of VHD that mostly affects people over 50 in high-income nations and is caused by age-related degenerative pathogeneses. There is growing evidence that degenerative VHD, including aortic stenosis (AS) and mitral regurgitation (MR), is influenced by immunological dysregulation and chronic inflammation.
Despite the growing evidence that RA and degenerative VHD share comparable inflammatory processes and risk factors, little is known about how they relate to one another. Thus, this study was set to examine the relationship between the risk of various forms of degenerative VHD and the prevalence of RA.
Cox proportional hazards models, with sequential adjustments for demographics, lifestyle factors, and comorbidities, were used to evaluate the relationship between prevalent RA and new-onset degenerative VHD.
Among 492 745 UK Biobank individuals who did not have VHD at baseline. 8 subtypes of degenerative VHD were identified in this study: tricuspid stenosis, tricuspid regurgitation, mitral stenosis, aortic regurgitation, aortic stenosis, pulmonary stenosis, and pulmonary regurgitation.
Over a median follow-up of 13.71 (interquartile range: 12.71–14.55) years, 359 instances of degenerative VHD were reported among individuals with RA (n=6673), whereas 13 518 cases were reported among people without RA (n=486–072) over a median follow-up of 13.78 (interquartile range: 12.96–14.51) years.
Aortic stenosis (hazard ratio [HR], 1.64 [95% CI, 1.40–1.92]), aortic regurgitation (HR, 1.69 [95% CI, 1.34–2.13]), and mitral regurgitation (HR, 1.54 [95% CI, 1.32–1.81]) were the three types of new-onset degenerative VHD that were significantly associated with RA after full adjustment.
No significant association was found between RA and other subtypes of degenerative VHD. Additionally, sex subgroup analysis showed that RA and sex interacted to increase the risk of aortic stenosis (P for interaction=0.02) and mitral regurgitation (P for interaction=0.04) in women.
Overall, while there was no significant correlation between RA and incident degenerative MS, TR, or PR, the results of this cohort analysis suggested that RA was linked to the development of degenerative AS, AR, and MR.
Furthermore, incident AS and MR may be more likely to occur in women with RA. In order to incorporate these findings into primary prevention in high-risk groups, more research is required.
Source:
Wang, Z., Lv, J., Qian, X., Li, Z., Yin, Z., Wang, C., Zhao, S., Gao, X., & Wu, Y. (2025). Association between rheumatoid arthritis and the risk of incident degenerative valvular heart disease: Evidence from a prospective cohort study. Journal of the American Heart Association,. https://doi.org/10.1161/JAHA.125.042025
Powered by WPeMatico
Powered by WPeMatico
Powered by WPeMatico
Powered by WPeMatico
