Topline results from the ATTAIN-2 trial have demonstrated that adults taking 36 mg orforglipron lost an average of 10.5% body weight at 72 weeks, with over half of participants on any dose achieving an HbA1c of 6.5% or lower. The oral GLP-1 demonstrated significant benefits for adults with obesity and type 2 diabetes.
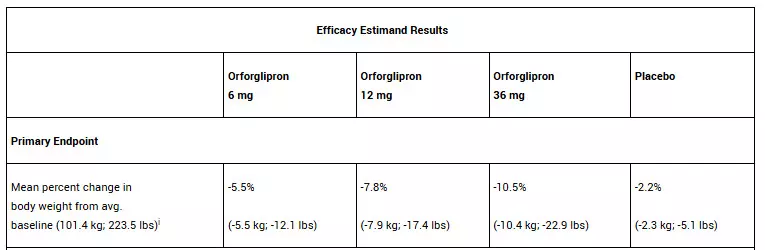
In the trial, all three doses of orforglipron met the primary and all key secondary endpoints, delivering significant weight loss, meaningful A1C reductions, and improvements in cardiometabolic risk factors at 72 weeks. For the primary endpoint, orforglipron 36 mg, taken once per day without food and water restrictions, lowered weight by an average of 10.5% (22.9 lbs) compared to 2.2% (5.1 lbs) with placebo using the efficacy estimand.1 With the completion of ATTAIN-2, Lilly now has the full clinical data package required to initiate global regulatory submissions for orforglipron.
“Based on my experience leading clinical trials in obesity and diabetes, these data show the potential for orforglipron to offer an efficacy, safety, and tolerability profile consistent with the injectable GLP-1 class,” said Louis J. Aronne, MD, FACP, DABOM, founder and Chair Emeritus of the American Board of Obesity Medicine, former president of The Obesity Society, Fellow of the American College of Physicians, and world-renowned obesity specialist. “Orforglipron could help health care providers expand treatment options for patients who prefer oral therapies without compromising clinical results.”
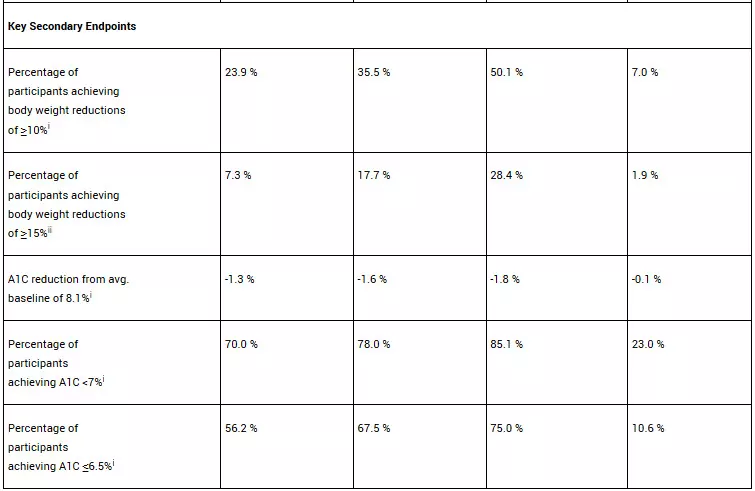
In the ATTAIN-2 trial, orforglipron met the primary endpoint of superior body weight reduction compared to placebo. Participants taking the highest dose of orforglipron lost an average of 22.9 lbs (10.5%) at 72 weeks using the efficacy estimand. In a key secondary endpoint, orforglipron lowered A1C by 1.3% to 1.8% from a baseline of 8.1% across doses. In another key secondary endpoint, 75% of participants taking the highest dose of orforglipron achieved an A1C ≤6.5%, which is at or below the American Diabetes Association’s definition of diabetes. Additionally, orforglipron showed clinically meaningful benefits across key cardiovascular risk factors, including non-HDL cholesterol, systolic blood pressure and triglycerides. In a pre-specified exploratory analysis, the highest dose of orforglipron reduced high-sensitivity C-reactive protein (hsCRP) levels, a marker of inflammation, by 50.6%.
iSuperiority test was adjusted for multiplicity with all three doses.
iiSuperiority test was adjusted for multiplicity with the 12 mg and 36 mg doses.
For the treatment-regimen estimand, each dose of orforglipron led to statistically significant improvements across the primary and all key secondary endpoints, including:
• Percent weight reduction: -5.1% (-5.3 kg; -11.7 lbs; 6 mg), -7.0% (-7.2 kg; -15.9 lbs; 12 mg), -9.6% (-9.6 kg; -21.2 lbs; 36 mg), -2.5% (-2.7 kg; -6.0 lbs; placebo)
• Percentage of participants achieving body weight reductions of ≥10%: 22.6% (6 mg), 31.2% (12 mg), 45.6% (36 mg), 9.0% (placebo)
• Percentage of participants achieving body weight reductions of ≥15%: 6.8% (6 mg), 14.4% (12 mg), 26.0% (36 mg), 3.0% (placebo)
• A1C reduction: -1.2% (6 mg), -1.5% (12 mg), -1.7% (36 mg), -0.5% (placebo)
• Percentage of participants achieving A1C <7%: 64.6% (6 mg), 75.9% (12 mg), 75.5% (36 mg), 30.5% (placebo)
• Percentage of participants achieving A1C ≤6.5%: 52.5% (6 mg), 57.6% (12 mg), 66.6% (36 mg), 15.4% (placebo)
“The ATTAIN-2 results reinforce the potential for orforglipron, as a once-daily oral, to deliver meaningful weight loss and A1C reduction, consistent with similar landmark trials for injectable GLP-1s,” said Kenneth Custer, Ph.D., Lilly executive vice president and president of Lilly Cardiometabolic Health. “With these positive data in hand, we are moving with urgency toward global regulatory submissions to potentially meet the needs of patients who are waiting. If approved, we are ready to offer a convenient, once-daily pill that can be scaled globally-removing barriers and redefining how obesity is treated around the world.”
The overall safety profile of orforglipron in ATTAIN-2 was consistent with the established GLP-1 receptor agonist class. The most commonly reported adverse events were gastrointestinal-related and generally mild-to-moderate in severity. The most common adverse events for participants treated with orforglipron (6 mg, 12 mg and 36 mg, respectively) were nausea (20.1%, 31.1% and 36.4%) vs. 8.4% with placebo, vomiting (12.8%, 20.2% and 23.1%) vs. 3.8% with placebo, diarrhea (21.3%, 24.8% and 27.4%) vs. 15.0% with placebo, constipation (17.7%, 21.1% and 22.4%) vs. 7.8% with placebo, and dyspepsia (9.1%, 15.4% and 10.9%) vs. 3.5% with placebo. Treatment discontinuation rates due to adverse events were 6.1% (6 mg), 10.6% (12 mg) and 10.6% (36 mg) for orforglipron vs. 4.6% with placebo. Overall treatment discontinuation rates were balanced across the treatment groups with 19.1% (6 mg), 22.3% (12 mg) and 20.5% (36 mg) for orforglipron vs. 20.0% with placebo. No hepatic safety signal was observed.
Detailed ATTAIN-2 results will be presented at a future medical meeting and published in a peer-reviewed journal.
About orforglipron
Orforglipron (or-for-GLIP-ron) is an investigational, once-daily small molecule (non-peptide) oral glucagon-like peptide-1 receptor agonist that can be taken any time of the day without restrictions on food and water intake.5 Orforglipron was discovered by Chugai Pharmaceutical Co., Ltd. and licensed by Lilly in 2018. Chugai and Lilly published the preclinical pharmacology data of this molecule together. Lilly is running Phase 3 studies on orforglipron for the treatment of type 2 diabetes and for weight management in adults with obesity or overweight with at least one weight-related medical problem. It is also being studied as a potential treatment for obstructive sleep apnea and hypertension in adults with obesity.
About ATTAIN-2 and ATTAIN clinical trial program
ATTAIN-2 (NCT05872620) is a Phase 3, 72-week, randomized, double-blind, placebo-controlled trial comparing the efficacy and safety of orforglipron 6 mg, 12 mg and 36 mg as monotherapy with placebo in adults with obesity or overweight and type 2 diabetes. The trial randomized over 1,600 participants across the U.S., Argentina, Australia, Brazil, China, Czechia, Germany, Greece, India, South Korea and Puerto Rico in a 1:1:1:2 ratio to receive either 6 mg, 12 mg or 36 mg orforglipron or placebo. The primary objective of the study was to demonstrate that orforglipron (6 mg, 12 mg, 36 mg) is superior to placebo in mean body weight change from baseline at 72 weeks in people with a BMI ≥27.0 kg/m² and type 2 diabetes who are on stable treatment with either diet/exercise alone or up to three oral antihyperglycemic medications. All participants receiving orforglipron started the study at a dose of 1 mg once-daily and then increased the dose in a step-wise approach at four-week intervals to their final randomized maintenance dose of 6 mg (via steps at 1 mg and 3 mg), 12 mg (via steps at 1 mg, 3 mg and 6 mg) or 36 mg (via steps at 1 mg, 3 mg, 6 mg, 12 mg and 24 mg). Dose reduction was only allowed for GI tolerability if other mitigations failed.
The ATTAIN Phase 3 global clinical development program for orforglipron has enrolled more than 4,500 people with obesity or overweight across two global registration trials.