Experts urge the medical profession to confront the global arms industry
Powered by WPeMatico
Powered by WPeMatico
Powered by WPeMatico
Powered by WPeMatico
Powered by WPeMatico
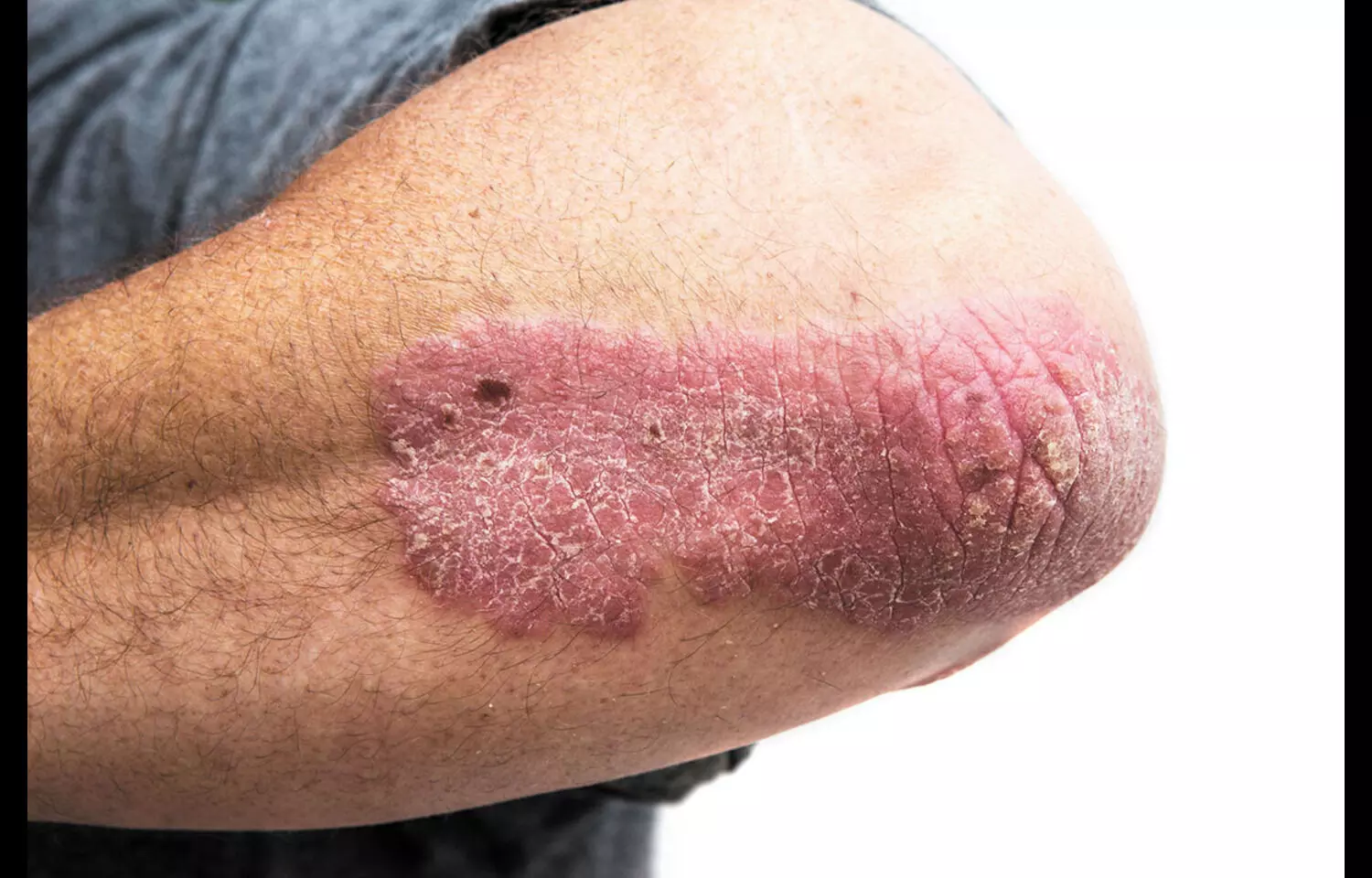
Adults with moderate to severe hidradenitis suppurativa (HS) treated with adalimumab face a significantly higher risk of serious infections compared to those with psoriasis, according to a new cohort study published in JAMA Dermatology. Researchers analyzed U.S. insurance claims data of 10,349 adults initiating adalimumab between 2017–2020—1,650 with HS and 8,699 with psoriasis. They found that the HS group had a 53% higher hazard of hospitalization for noncutaneous infections (HR 1.53; 95% CI 1.34–1.86) after adjusting for confounders. Rates of sepsis (IRR 2.07; 95% CI 1.35–3.12) and genitourinary infections (IRR 2.22; 95% CI 1.22–3.86) were more than double in the HS cohort. HS patients also had a 28% higher odds of prolonged hospital stays (OR 1.28; 95% CI 1.13–1.45). Notably, HS patients were younger (mean age 36.2 vs. 46.5 years), more often female (77% vs. 50%), and had higher rates of obesity, Crohn’s disease, anxiety, and depression. The findings suggest that HS patients face a distinct infectious risk profile under immunosuppressive therapy compared to those with psoriasis. Researchers highlight the need for further studies to understand how disease severity, treatment regimens, and preventive strategies could mitigate this elevated infection risk. Until then, clinicians should remain vigilant when managing HS patients with adalimumab and consider close monitoring and tailored infection-prevention strategies.
Keywords: adalimumab, hidradenitis suppurativa, psoriasis, infection risk, serious infection, JAMA Dermatology, sepsis, genitourinary infection, hospitalization, cohort study
Powered by WPeMatico

Omega fatty acids could protect against Alzheimer’s disease in women, new research has found.
Analysis of lipids – fat molecules that perform many essential functions in the body – in the blood found there was a noticeable loss of unsaturated fats, such as those that contain omega fatty acids, in the blood of women with Alzheimer’s disease compared to healthy women.
Scientists found no significant difference in the same lipid molecule composition in men with Alzheimer’s disease compared to healthy men, which suggests that those lipids have a different role in the disease according to sex. Fats perform important roles in maintaining a healthy brain, so this study could indicate why more women are diagnosed with the disease.
The study, published today in Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association by scientists from King’s College London and Queen Mary University London, is the first to reveal the important role lipids could have in the risk for Alzheimer’s between the sexes.
Senior author Dr Cristina Legido-Quigley, from King’s College London, said: “Women are disproportionately impacted by Alzheimer’s Disease and are more often diagnosed with the disease than men after the age of 80. One of the most surprising things we saw when looking at the different sexes was that there was no difference in these lipids in healthy and cognitively impaired men, but for women this picture was completely different. The study reveals that Alzheimer’s lipid biology is different between the sexes, opening new avenues for research.”
The scientists took plasma samples from 841 participants who had Alzheimer’s Disease, mild cognitive impairment and cognitively health controls and and were measured for brain inflammation and damage.
They used mass spectrometry to analyse the 700 individual lipids in the blood. Lipids are a group of many molecules. Saturated lipids are generally considered as ‘unhealthy’ or ‘bad’ lipids, while unsaturated lipid, which sometime contains omega fatty acids, are generally considered ‘healthy’.
Scientists saw a steep increase in lipids with saturation – the ‘unhealthy lipids’ – in women with Alzheimer’s compared to the healthy group. The lipids with attached omega fatty acids were the most decreased in the Alzheimer’s group.
Now, the scientists say there is a statistical indication that there is a causal link between Alzheimer’s Disease and fatty acids. But a clinical trial is necessary to confirm the link.
Dr Legido-Quigley added: “Our study suggests that women should make sure they are getting omega fatty acids in their diet – through fatty fish or via supplements. However, we need clinical trials to determine if shifting the lipid composition can influence the biological trajectory of Alzheimer’s Disease.”
Dr Asger Wretlind, first author of the study from King’s College London, said: “Scientists have known for some time that more women than men are diagnosed with Alzheimer’s disease. Although this still warrants further research, we were able to detect biological differences in lipids between the sexes in a large cohort, and show the importance of lipids containing omegas in the blood, which has not been done before. The results are very striking and now we are looking at how early in life this change occurs in women.”
Dr Julia Dudley, Head of Research at Alzheimer’s Research UK says: “In the UK, two in three people living with dementia are women. This could be linked to living longer, or other risk factors like social isolation, education, or hormonal changes from the menopause being at play.
“While this study shows that women with Alzheimer’s had lower levels of some unsaturated fats compared with men, further work is needed. This includes understanding the mechanisms behind this difference and finding out if lifestyle changes, including diet could have a role. Future research should also be carried out in a more ethnically diverse population to see if the same effect is seen.
“Understanding how the disease works differently in women could help doctors tailor future treatments and health advice. Alzheimer’s Research UK is proud to be funding this work that will bring us a step closer to a cure.”
Reference:
Wretlind, A., et al. (2025). Lipid profiling reveals unsaturated lipid reduction in women with Alzheimer’s disease. Alzheimer’s & Dementia. doi.org/10.1002/alz.70512.
Powered by WPeMatico

A new study published in the journal of medRxiv revealed that individuals with higher levels of physical activity, as measured by wearable devices, experienced modest but statistically significant reductions in new-onset gout. The benefit was observed regardless of whether exercise was spread throughout the week or concentrated on weekends. As the findings are currently from a preprint, they remain preliminary pending peer-reviewed publication.
The study analyzed a total of 97,474 gout-free adults who wore wrist-based accelerometers for 7 days to track physical activity. The participants were grouped into 3 categories: Inactive (fewer than 150 minutes per week of moderate-to-vigorous physical activity [MVPA]); Weekend Warrior (WW) (at least 150 minutes of MVPA, with more than half compressed into one or two days); and Regularly Active (RA) (at least 150 minutes of MVPA spread more evenly across the week).
Over a median follow-up of 8 years, 905 participants developed gout. When compared to inactive individuals (36,785 people), the individuals in the RA group (20,298 people) showed a 25% lower risk of gout, while those in the WW group (40,391 people) had a 16% lower risk. Specifically, hazard ratios (HRs) were 0.75 for RA and 0.84 for WW, indicating a statistically significant protective effect for both.
However, when this research adjusted for total MVPA volume, the difference between RA and WW groups disappeared. This suggests that the amount of exercise matters more than whether it is spread throughout the week or concentrated on weekends. Further analyses using different definitions of weekend warrior activity, excluding early gout cases, and adjusting for genetic predisposition all confirmed the robustness of the findings.
When this research considered the genetic risk of participants for gout using polygenic risk scores, the benefits of physical activity remained consistent across all genetic profiles. In other words, both regular exercisers and weekend warriors enjoyed protective effects regardless of whether they had a high or low inherited risk for the disease.
The study emphasized that even those with busy lifestyles who can only fit exercise into weekends may still gain substantial health benefits. Gout, a form of inflammatory arthritis characterized by sudden and painful joint swelling, has been rising globally due to lifestyle changes and increasing obesity rates. Preventive strategies are therefore a public health priority.
Overall, both consistent weekly exercise and concentrated weekend workouts can meaningfully reduce gout risk, provided that individuals meet the recommended threshold of at least 150 minutes of moderate-to-vigorous activity per week.
Source:
Wang, Q., Liu, Y., & Zhu, B. (2025). Accelerometer-derived physical activity volume, not pattern, correlates with reduced gout incidence in a UK prospective cohort. In medRxiv. https://doi.org/10.1101/2025.08.24.25334337
Powered by WPeMatico
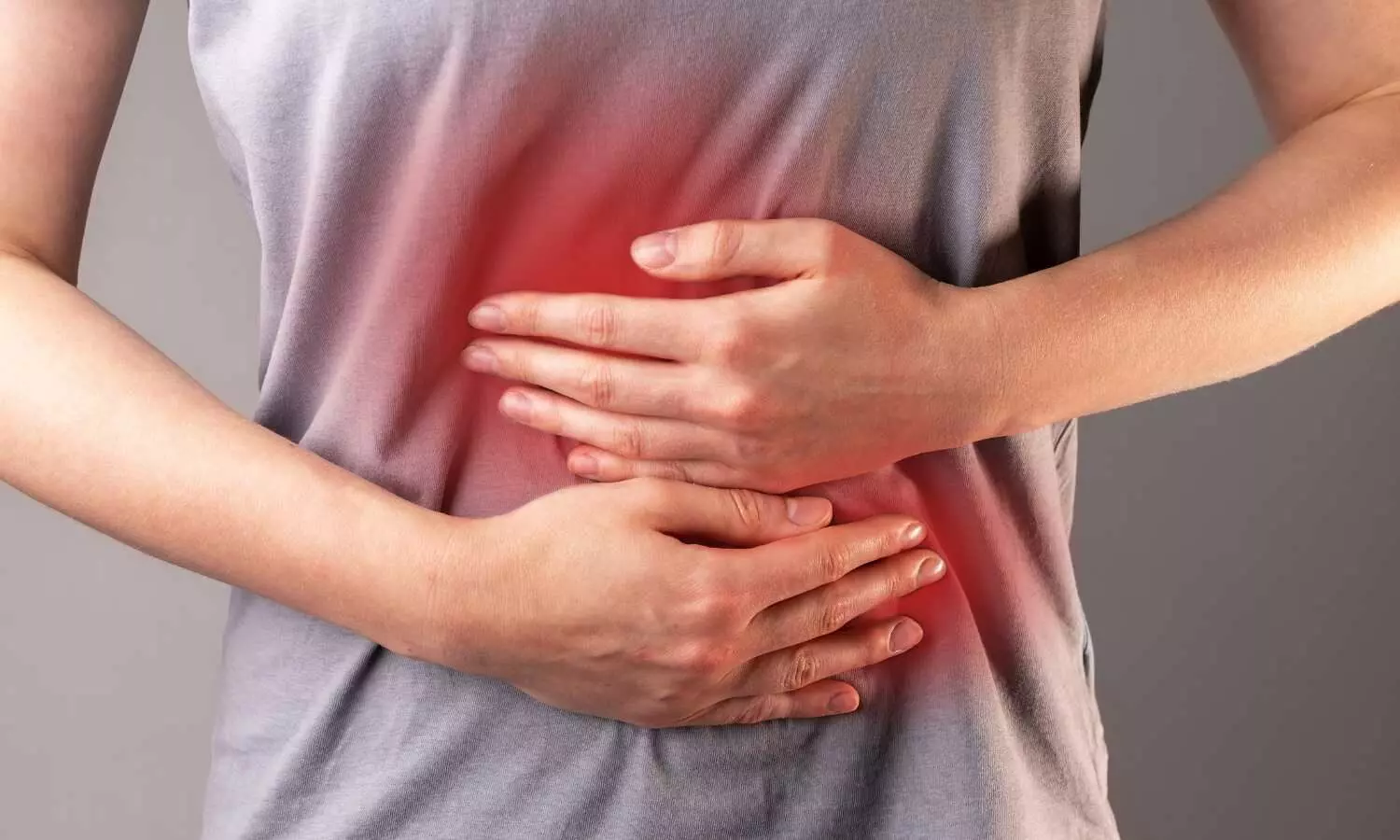
Imbalanced autonomic function, characterized by reduced vagal activity and sympathetic dominance, is increasingly recognized in various gastrointestinal (GI) disorders. The vagus nerve, a key component of the parasympathetic nervous system, plays a critical role in regulating upper GI motility, inflammation, and pain perception. Transcutaneous vagal nerve stimulation (tVNS) offers a non-invasive method to modulate vagal activity, presenting a promising therapeutic approach for GI conditions. This review synthesizes evidence from clinical trials on the efficacy of tVNS-including transcutaneous auricular (taVNS), cervical (tcVNS), and percutaneous electrical nerve field stimulation (PENFS)-in managing abdominal pain, improving GI symptoms, and enhancing motility in disorders such as functional dyspepsia (FD), gastroparesis, irritable bowel syndrome (IBS), and inflammatory bowel disease (IBD). tVNS demonstrates significant potential in alleviating symptoms and restoring autonomic balance through anti-inflammatory, anti-nociceptive, and prokinetic mechanisms. However, larger, standardized trials are needed to optimize stimulation parameters and confirm clinical efficacy.
The vagus nerve is the longest cranial nerve, innervating thoracic and abdominal organs and facilitating bidirectional communication between the brain and the gut via afferent (80%) and efferent (20%) fibers. It regulates essential GI functions—motility, secretion, satiety, and inflammation—through the dorsal vagal complex in the brainstem. Autonomic imbalance, marked by reduced vagal tone, is implicated in various GI disorders, including FD, gastroparesis, IBS, and IBD. Traditional invasive VNS, while effective, is limited by its cost, surgical risks, and side effects. Non-invasive tVNS methods—taVNS, tcVNS, and PENFS—offer safer, patient-friendly alternatives by stimulating superficial vagal branches through the skin or ear. This review evaluates the clinical effects and mechanisms of tVNS in GI disorders, drawing on evidence from randomized trials and preclinical studies.
Chronic abdominal pain is a hallmark of disorders of gut-brain interaction (DGBIs), affecting up to 25% of the U.S. population. Six clinical trials have explored tVNS for pain relief:
• taVNS in FD: A multicenter RCT with 330 FD patients showed that 4 weeks of taVNS (10 Hz or 25 Hz) significantly reduced stomach pain compared to sham (75–82.8% vs. 61.5% response). Improvements were also seen in bloating and fullness.
• taVNS in IBS-C: A study of 42 patients reported a 64% reduction in abdominal pain and tripled weekly bowel movements after 4 weeks of taVNS.
• tcVNS in Gastroparesis: An open-label trial using a handheld stimulator (gammaCore) improved pain and GI symptoms in 15 patients, though autonomic function remained unchanged.
• PENFS in Adolescents: RCTs demonstrated sustained reductions in worst abdominal pain and improved well-being in IBS patients after 3 weeks of PENFS.
One pilot study in 22 pediatric IBD patients (Crohn’s disease and ulcerative colitis) investigated taVNS (20 Hz). After 16 weeks, 50% of CD and 33% of UC patients achieved clinical remission, and 64.7% showed ≥50% reduction in fecal calprotectin, indicating anti-inflammatory effects. Limitations include small sample size and lack of prolonged sham control.
GI dysmotility contributes significantly to symptoms in DGBIs. Three trials evaluated tVNS:
• Esophageal Motility: taVNS enhanced upper and lower esophageal sphincter pressures in patients with laryngopharyngeal reflux.
• Gastric Accommodation and Slow Waves: In FD patients, taVNS improved gastric accommodation and normalized slow waves.
• Colonic and Anorectal Function: In IBS-C, taVNS enhanced rectal sensation and improved reflex activity, alongside reduced proinflammatory cytokines (TNF-α, IL-6).
tVNS exerts therapeutic effects through multiple pathways:
• Anti-nociceptive Effects: Modulates visceral hypersensitivity via vagal afferents to the NTS, influencing pain-processing regions (e.g., hypothalamus, amygdala).
• Anti-inflammatory Effects: Activates the cholinergic anti-inflammatory pathway, where acetylcholine binds α7nAChR on macrophages, suppressing TNF-α, IL-6, and IL-1β.
• Prokinetic Effects: Enhances vagal efferent activity, improving GI slow waves, accommodation, and transit. HRV studies confirm increased parasympathetic tone.
• Central Mechanisms: fMRI studies in migraine show taVNS activates NTS and modulates pain networks; similar mechanisms are hypothesized for GI disorders.
Current evidence is limited by small sample sizes, variability in stimulation parameters (frequency, duration, site), and lack of consensus on optimal protocols. Preclinical studies suggest higher frequencies (e.g., 100 Hz) may be more effective for pain, while lower frequencies (25 Hz) improve motility, but clinical translation is needed. Future research should focus on
• Large-scale, multicenter RCTs.
• Standardization of stimulation protocols.
• Integration with multi-omics and neuroimaging.
• Personalized approaches based on autonomic profiling.
Transcutaneous VNS represents a novel, non-invasive, and safe therapy for GI disorders, with demonstrated benefits in pain relief, inflammation reduction, and motility enhancement. Its mechanisms involve modulation of vagal pathways, central pain processing, and immune responses. While promising, broader clinical adoption requires further high-quality studies to establish optimized parameters and confirm efficacy across diverse GI conditions.
Reference:
Yin J. Transcutaneous Vagal Nerve Stimulation for Gastrointestinal Disorders. J Transl Gastroenterol. 2025;3(2):93-99. doi: 10.14218/JTG.2025.00024.
Powered by WPeMatico

Breastfeeding within the first 4 to 6 months of life has been identified by researchers as being associated with a reduced risk of central precocious puberty (CPP) in children of both genders. CPP is the condition of early onset of puberty at an unnatural age, and it has been on the rise globally, triggering concerns about long-term consequences such as metabolic disorders, psychological distress, and enhanced risk of various diseases later in life. This study was published in JAMA Network Open by Yunsoo C. and colleagues.
It was a big retrospective cohort analysis that used data from the South Korean National Health Insurance Service Database from January 1, 2007, to December 31, 2020. There were 322,731 children who received regular health checkups at 4 to 6 months and once more at 66 to 71 months. Children with comorbidities, those who died during the follow-up period, those with missing data, or those diagnosed with CPP before age 6 years were excluded.
Infant feeding patterns were measured with caregiver-completed questionnaires during the 4–6-month visit. Children were categorized as being exclusively breastfed (46.0%), formula-fed (34.9%), or mixed-fed (19.1%). The main outcome was incidence of CPP, defined by diagnostic codes and verified with treatment with gonadotropin-releasing hormone agonists. Multivariable Cox proportional hazards models were applied to estimate adjusted hazard ratios (AHRs), and mediation analysis was conducted to test whether childhood overweight or obesity accounted for some of the association.
Results
The results indicated that the greatest risk of CPP was linked with formula feeding, followed by mixed feeding, relative to exclusive breastfeeding.
For boys, formula feeding raised the risk of CPP by 16% (AHR, 1.16; 95% CI, 1.10–1.21), whereas mixed feeding raised the risk by 14% (AHR, 1.14; 95% CI, 1.07–1.20).
The risks were even greater in girls. Formula feeding was linked to a 60% higher risk (AHR, 1.60; 95% CI, 1.24–2.06), and mixed feeding to a 45% higher risk (AHR, 1.45; 95% CI, 1.07–1.97).
Mediation analysis revealed that prepubertal adiposity mediated a part of the association. Excess weight in childhood explained 7.2% of the formula–CPP relationship in boys (bootstrap 95% CI, 4.5%–12.1%) and 17.8% in girls (bootstrap 95% CI, 6.6%–30.0%).
This large South Korean national study identified that sole breastfeeding during the first 4–6 months of life is linked to a reduced risk of central precocious puberty in both girls and boys, but formula feeding and mixed feeding were associated with much increased risks. Encouraging breastfeeding and the prevention of childhood obesity may be crucial in reducing the burden of early puberty.
Reference:
Choe Y, Ryu S, Choi J, et al. Breastfeeding, Prepubertal Adiposity, and Development of Precocious Puberty. JAMA Netw Open. 2025;8(8):e2527455. doi:10.1001/jamanetworkopen.2025.27455
Powered by WPeMatico
Powered by WPeMatico
