Cultivating compassion in children can lead to healthier eating habits
Powered by WPeMatico
Powered by WPeMatico
Powered by WPeMatico
Australia: A case report published in BMC Nephrology by Seethalakshmi Viswanathan and colleagues from the Tissue Pathology & Diagnostic Oncology, Institute of Clinical Pathology and Medical Research, Westmead Hospital, Australia, has highlighted the severe renal consequences of untreated paroxysmal nocturnal haemoglobinuria (PNH) in a patient with a history of aplastic anaemia (AA). The report underlines the importance of vigilant follow-up and timely intervention in patients with PNH clones to prevent long-term complications such as chronic renal haemosiderosis.
PNH is a rare, life-threatening disorder marked by intravascular haemolysis, anaemia, and thrombosis. Kidney involvement often results from chronic haemosiderin deposition or microvascular thrombosis. In this case, the authors describe a 74-year-old woman previously treated for AA who later developed an expanding PNH clone but did not receive complement inhibitor therapy before being lost to follow-up.
The patient was admitted in April 2024 with fever, malaise, severe haemolytic anaemia, and acute kidney injury in the setting of Escherichia coli sepsis. Laboratory workup confirmed renal dysfunction, elevated serum creatinine, and a large PNH clone involving over 80% of neutrophils and monocytes. Despite stabilisation of haemolysis after infection control and red blood cell transfusions, her kidney function continued to deteriorate.
A renal biopsy performed on day 33 revealed extensive brown pigment deposits in most proximal tubules, confirmed as haemosiderin on Perls’ Prussian blue staining, along with severe acute tubular injury. Chronic damage, including interstitial fibrosis, tubular atrophy, and inflammation, was also noted. The findings supported a diagnosis of acute tubular injury on a background of chronic kidney disease, largely attributable to ongoing intravascular haemolysis from untreated PNH, with possible contributions from hypertension and diabetes.
Her hospital course was further complicated by multiple cerebral cortical venous thromboses and recurrent E. coli urosepsis. Persistent fevers and elevated inflammatory markers prompted further evaluation, which revealed thoracic aortitis. She was diagnosed with giant cell arteritis (GCA) and responded rapidly to high-dose corticosteroids, with improvement in inflammatory markers, haemoglobin, and renal function.
By discharge, her creatinine had stabilised at 160–170 µmol/L, and she was initiated on ravulizumab, a C5 complement inhibitor, once the risk of sepsis was reduced. Six months later, both her haemolysis and kidney function remained stable.
The authors emphasise that this case illustrates the severe renal complications that can arise from prolonged, uncontrolled haemolysis in PNH, particularly in patients with a prior history of AA. It also underscores the need for regular surveillance in AA survivors for the emergence or progression of PNH clones and the importance of addressing haemolytic triggers promptly. The rare coexistence of PNH with GCA in this patient further highlights the value of comprehensive diagnostic evaluation in complex presentations.
Reference:
Goh, Z.Z., Tang, K., Chau, K. et al. Severe renal haemosiderosis in a patient with untreated paroxysmal nocturnal haemoglobinuria: a case report. BMC Nephrol 26, 435 (2025). https://doi.org/10.1186/s12882-025-04343-5
Powered by WPeMatico
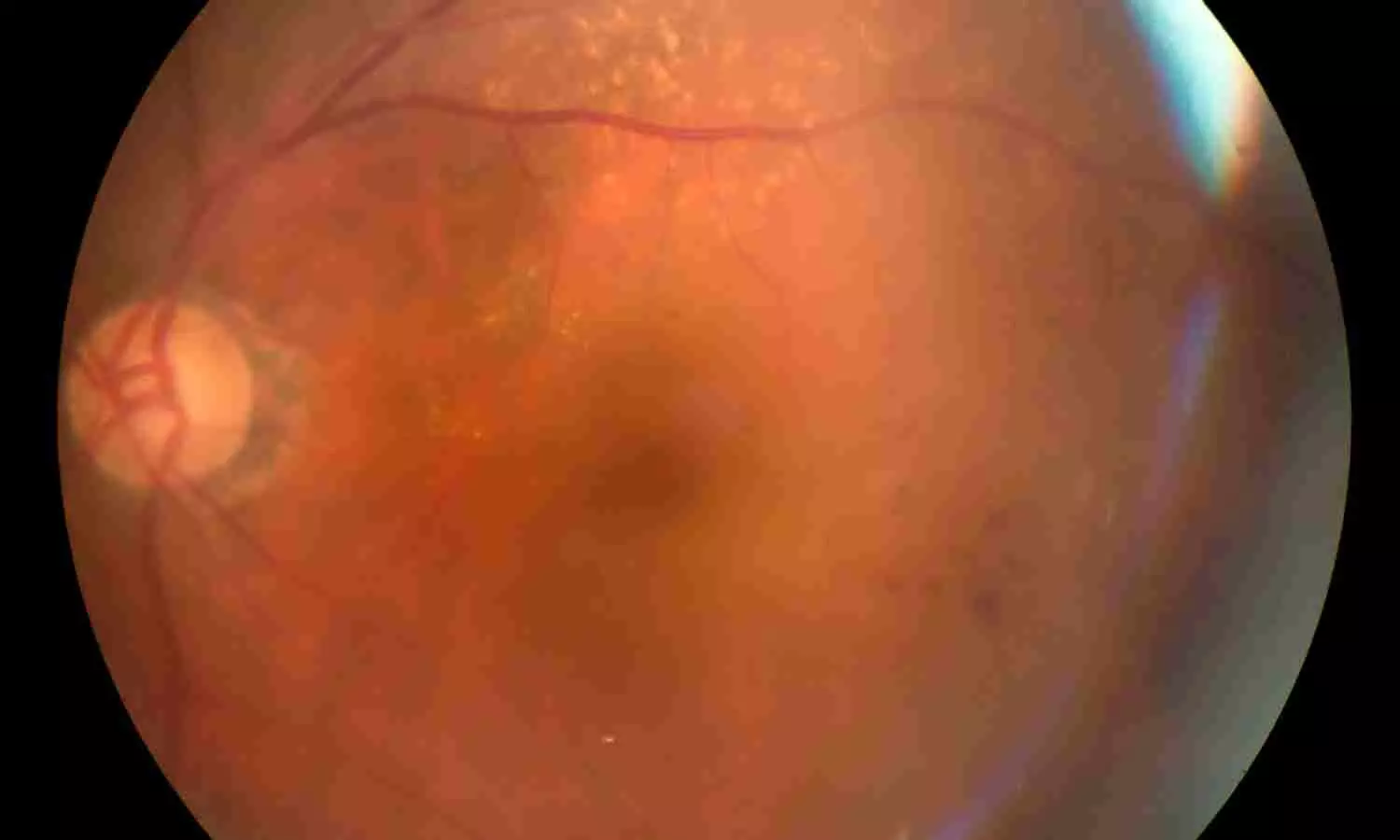
Researchers have determined that the red cell distribution width-to-albumin ratio (RAR), a composite biomarker of systemic inflammation and nutritional status, is linked to the risk of retinopathy in US adults over age 40. The new research identifies that the association between RAR and retinopathy is not linear and suggests that RAR may be a valuable tool for identifying patients at higher risk. The study was published in the Journal of Multidisciplinary Healthcare by Gu J. and colleagues.
Diabetic retinopathy, the diabetic complication most frequently linked to diabetes, was quantitated in diabetic and nondiabetic subjects through standardized fundus photographs and the ETDRS grading system. The information came from the National Health and Nutrition Examination Survey (NHANES) 2005–2008, which comprised a nationally representative sample of 4,753 adults aged 40 years and older in the United States.
This was a cross-sectional study using NHANES data from 2005 to 2008. A total of 4,753 adults aged 40 and older were included. Retinopathy was determined through retinal photographs evaluated according to the ETDRS grading protocol. RAR was estimated from laboratory data, and participants were divided into quartiles according to RAR levels. To examine the nature of the relationship between RAR and retinopathy, researchers employed generalized additive models to assess nonlinear trends, and a two-piecewise linear regression model to detect the point of inflection at which the relation changed.
Key Findings
The univariate analysis disclosed a very pronounced increase in retinopathy prevalence in association with increasing quartiles of RAR (P < 0.001).
A clear nonlinear relationship between RAR and retinopathy risk was observed, with an inflection point at a RAR value of 3.14.
Below the inflection point (RAR < 3.14): With each 1-unit increase in RAR, there were 2.69 times greater odds of retinopathy (OR = 3.69; 95% CI: 1.37–9.96).
Above the inflection point (RAR > 3.14): The relationship flattened, with a non-significant rise in odds (OR = 1.14; 95% CI: 0.60–2.14).
Highest vs. lowest RAR quartile: Individuals with the highest quartile had 56% higher odds of retinopathy than individuals in the lowest quartile (OR = 1.56; 95% CI: 1.16–2.11).
This population-based research found that the RAR has a nonlinear correlation with the risk of retinopathy in U.S. adults aged 40 and above. The risk grew disproportionately large below an RAR value of 3.14, beyond which the association plateaued. These results uphold the promise of RAR as a simple, low-cost biomarker for retinopathy risk stratification, particularly in primary care and public health settings.
Reference:
Powered by WPeMatico
Significant risk factors associated with maternal and neonatal health stem from spontaneous preterm labor (PTL), which accounts for approximately 70% of preterm births. Current biomarkers lack prediction reliability, highlighting the need for novel, non-invasive indicators. Emerging research on tRNA-derived small RNAs (tsRNAs), however, shows promise in this exploration yet remains underinvestigated in relation to PTL.
Recent study analyzed publicly available small RNA sequencing datasets (PRJNA415953 and PRJNA428989). The analysis included peripheral blood plasma and exosomes collected from women diagnosed with spontaneous PTL and term controls during early pregnancy (12±1 weeks of gestation). The tsRNA profiles were assessed for differential expression using normalized reads per million (RPM) and subjected to statistical analysis (Wilcoxon tests) against established thresholds (p ≤ 0.05, |log2(Fold Change)| ≥ 1). Specifically, tsRNA1 (tRNA-Gly-GCC-5p-tRF-921) was identified and subsequently validated through functional assays, RT-qPCR, and transcriptomic profiling of human HTR-8/SVneo trophoblast cells.
Key Results:
• Differential Expression: Analysis showed multiple tsRNAs were significantly upregulated in the samples from PTL cases compared to controls, particularly in plasma extracellular vesicles (EVs). This included 32 upregulated tsRNAs in whole blood and associated variations in terms of specific tRF types.
• Functional Role of tsRNA1: Overexpression of tsRNA1 in trophoblast cells induced apoptosis and inhibited critical processes like cell migration, adhesion, and invasion, pivotal for placental development.
• Epigenetic Modulation: Transcriptomic analysis indicated that tsRNA1 modulates genes involved in DNA methylation (in particular TET1 and DNMT1), suggesting tsRNA1’s role in establishing a hypermethylated state, which could contribute to impaired trophoblast function and further the risk of PTL.
Limitations:
• The primary use of the HTR-8/SVneo cell line may not fully replicate the behavior of primary extravillous trophoblasts (EVTs), necessitating validation in more physiologically relevant systems.
• There was no direct evidence provided concerning metabolic changes or validation methods for assessing extracellular vesicle purity. The absence of quantitative data, such as nanoparticle tracking analysis, limits the interpretation of the isolated vesicle types.
The findings position tsRNA1 and tsRNA3 as potential biomarkers for early prediction of PTL, emphasizing the necessity for further validation in larger cohorts and exploration of their mechanistic role in pregnancy complications.
Key Points –
Spontaneous Preterm Labor Contribution: Spontaneous preterm labor (PTL) is responsible for approximately 70% of preterm births, highlighting the urgency in identifying reliable biomarkers for early prediction and clinical intervention.
Biomarker Discovery and tsRNAs: Emerging research points to tRNA-derived small RNAs (tsRNAs) as promising candidates for novel, non-invasive biomarkers linked to PTL. However, their application in this area remains underexplored.
Differential tsRNA Expression in PTL: Analysis of publicly available small RNA sequencing datasets revealed significant upregulation of several tsRNAs in peripheral blood plasma and exosomes from women diagnosed with PTL. Specifically, 32 tsRNAs were identified as upregulated in whole blood, with notable variations in specific tRNA fragment types.
Functional Implications of tsRNA1: The overexpression of tsRNA1 (tRNA-Gly-GCC-5p-tRF-921) in trophoblast cells induced apoptosis and negatively impacted vital cellular processes, including migration, adhesion, and invasion, which are critical for healthy placental development.
Epigenetic Influence on Trophoblast Function: Transcriptomic profiling indicated that tsRNA1 influences the expression of genes associated with DNA methylation, such as TET1 and DNMT1, leading to a hypermethylated state that may compromise trophoblast function, thus increasing the risk of PTL.
Research Limitations and Future Directions: The reliance on the HTR-8/SVneo cell line may not accurately mimic primary extravillous trophoblast behavior, underscoring the need for validation in more relevant models. Additionally, a lack of quantitative data regarding extracellular vesicle purity limits the study’s conclusions, indicating that larger cohort studies are necessary to validate tsRNA1 and tsRNA3 as biomarkers and to elucidate their mechanistic roles in PTL.
Reference –
Xinrui Sun et al. (2025). Maternal Plasma Extracellular Vesicles TsRNA As Potential Biomarkers For Assessing Preterm Labor Risk. *BMC Pregnancy And Childbirth*, 25. https://doi.org/10.1186/s12884-025-07672-3.
Powered by WPeMatico
A new study published in The Bone and Joint Journal showed that for the diagnosis of infections in the lower limbs associated with refractory fractures, positron emission tomography (PET)/CT is a useful diagnostic technique.
A significant clinical problem is fracture-related infection (FRI), which frequently arises following fractures or surgical operations that specifically impact the lower limbs. FRI is still difficult to diagnose, especially in chronic patients that frequently exhibit a wide range of unusual clinical symptoms. Even if a consensus has been presented by the AO international expert group, there are still no reliable and consistent diagnostic techniques, particularly when contrasted with the recognized standards for periprosthetic joint infections (PJIs).
By determining ideal maximum standardized uptake value (SUVmax) thresholds and examining sub-group-specific diagnostic performance, this study seeks to assess the diagnostic accuracy of 18F-FDG PET/CT for refractory FRIs in the lower limbs.
A total of 429 PET/CT images from a tertiary orthopaedic center conducted between November 2016 and October 2021 were included in this retrospective analysis. Included were patients with probable refractory FRI, which is defined as an infection that has persisted after at least 2 previous therapies.
Microbiological cultures, histological examination, intraoperative results, and follow-up data were all included in the reference standard. Receiver operating characteristic curve analysis was used to compute diagnostic performance parameters, such as sensitivity, specificity, and area under the curve (AUC). The patients were classified in sub-group analyses according to the time since prior surgery and the clinical presentation.
AUC for PET/CT was 0.84, indicating strong diagnostic performance. With a sensitivity of 70.7% and specificity of 85.6%, the ideal SUVmax threshold was 4.75. According to subgroup studies, customized thresholds increased diagnosis accuracy; at a threshold of 5.05., the “No signs + Early phase” group had the best accuracy (87.5%) and specificity (89.4%).
By separating infection from leftover metabolic activity, a more stringent cut-off of 3.95 in the “No signs + Over phase” group, on the other hand, reduced overdiagnosis. Given the difficulties in identifying chronic infections, the “Over phase” group had the highest false-positive rate (42.4%) and the best specificity (90.1%) at a cut-off of 4.65. These results highlight how SUVmax thresholds vary depending on the clinical context.
Overall, 18F-FDG With stratified SUVmax criteria that improve diagnosis accuracy dependent on the period since prior surgery and symptom manifestation, PET/CT is a dependable diagnostic tool for refractory FRI. By enhancing infection control and lowering recurrence rates, PET/CT proves to be cost-effective over the long run, even with its high initial cost.
Source:
Cai, W., Lu, Y., Ren, Z., Zhang, Y., Cheng, P., Chen, X., Han, P., & Xu, Z. (2025). Real-world experience of the role of 18F-fluorodeoxyglucose positron emission tomography/CT refractory fracture-related infection on lower limbs : a retrospective diagnostic study: A retrospective diagnostic study. The Bone & Joint Journal, 107-B(8), 846–856. https://doi.org/10.1302/0301-620X.107B8.BJJ-2024-1158.R2
Powered by WPeMatico

Recent study evaluated maternal serum neuroserpin concentrations in pregnancies affected by preeclampsia and their relationship with disease severity, considering neuroserpin’s roles in anti-inflammation and neuroprotection. This prospective case-control investigation was conducted from September 2024 to January 2025 at a tertiary referral hospital in Ankara, Türkiye, with a total of 88 participants: 44 diagnosed with preeclampsia and 44 healthy gestational-age-matched controls. ### Methodology Participants comprised pregnant women aged 21 to 45 with singleton pregnancies, diagnosed with preeclampsia based on the American College of Obstetricians and Gynecologists (ACOG) criteria. Exclusion criteria included multiple gestations, existing maternal comorbidities, substance abuse, prior corticosteroid treatment, and fetal anomalies. Serum samples (≥ 3 mL) were collected post-fasting and preserved at -80 °C until analysis. Neuroserpin levels were quantified using a Human NSP ELISA Kit via the sandwich ELISA method, with statistical significance evaluated through various tests (e.g., Student’s t-test, Mann-Whitney U test) and assessed using receiver operating characteristic (ROC) curve analysis for diagnostic efficacy. ### Key Results
The results demonstrated significantly reduced serum neuroserpin levels in women with preeclampsia compared to the control group (p = 0.018). Furthermore, women with severe preeclampsia (Preeclampsia-SF) exhibited even lower neuroserpin levels, indicating a potential link between neuroserpin reduction and disease severity. Notably, these variations in neuroserpin levels occurred independently of maternal demographic factors, such as age and BMI, enhancing its potential as a biomarker for preeclampsia identification.
Diagnostic Analysis
ROC analysis established that a neuroserpin level threshold of ≤ 22.95 ng/mL could identify preeclampsia with a sensitivity of 86.4% and specificity of 43.2%. For severe cases, a threshold of ≤ 14.7 ng/mL produced a sensitivity of 50.0% and a specificity of 96.7%. These outcomes indicate that neuroserpin could function as a valuable diagnostic instrument for evaluating both the presence and severity of preeclampsia.
Neuroserpin Properties –
Neuroserpin, a serine protease inhibitor secreted mainly by neurons and monocytes, possesses known anti-inflammatory properties that could alleviate the endothelial and inflammatory dysfunctions associated with preeclampsia. Under normal physiological conditions, it is thought to mitigate extracellular damage, particularly in ischemic situations, which may be vital for preserving vascular integrity during pregnancy. These findings suggest that decreased neuroserpin levels in preeclamptic pregnancies may exacerbate unregulated vascular inflammation, thereby worsening endothelial dysfunction.
Study Limitations
Limitations of this study include its relatively small sample size and its focus on a single center, which may limit the generalizability of the findings. Additionally, the cross-sectional design does not permit longitudinal tracking of neuroserpin levels throughout pregnancy or during postpartum recovery. ### Conclusion
Implications
In conclusion, lower maternal serum neuroserpin levels are significantly linked to both the presence and severity of preeclampsia, indicating its potential as a biomarker for assessing disease burden and inflammatory activity. Future multi-center, longitudinal studies could further confirm these results and investigate the clinical implications of neuroserpin in predicting and managing preeclampsia.
The key points from the research paper are: 1. Maternal serum neuroserpin levels were significantly lower in women diagnosed with preeclampsia compared to healthy pregnant controls. Moreover, neuroserpin levels were further decreased in those with severe preeclampsia, suggesting a potential role of neuroserpin not only in the presence but also in the progression of the disease. 2. The observed suppression of neuroserpin in preeclamptic pregnancies may reflect impaired physiological upregulation in response to inflammatory vascular stress. In healthy pregnancies, neuroserpin expression may serve as a compensatory mechanism to maintain endothelial integrity, and its reduction in preeclampsia could facilitate unchecked vascular inflammation and endothelial dysfunction. 3. Receiver operating characteristic (ROC) curve analysis showed that a serum neuroserpin cutoff value of ≤ 22.95 ng/mL had a sensitivity of 86.4% and a specificity of 43.2% for identifying preeclampsia, while a cutoff of ≤ 14.7 ng/mL had a sensitivity of 50.0% and a specificity of 96.7% for identifying severe preeclampsia. These findings suggest that neuroserpin may serve as a candidate biomarker reflecting disease burden and inflammatory activity in preeclampsia.
Reference –
Belgin Savran Ucok et al. (2025). Investigation Of Serum Neuroserpin Levels In Pregnant Women Diagnosed With Pre-Eclampsia: A Prospective Case-Control Study. *BMC Pregnancy And Childbirth*, 25. https://doi.org/10.1186/s12884-025-07673-2.
Powered by WPeMatico

The FDA has approved Ajovy (fremanezumab-vfrm) for preventive treatment of episodic migraine in pediatric patients aged 6 to 17 years who weigh at least 45 kg.
With this approval, AJOVY becomes the first and only calcitonin gene-related peptide (CGRP) antagonist indicated for the preventive treatment of episodic migraine in pediatric patients and migraine in adults, marking a meaningful advancement in expanding preventive treatment options for those living with migraine.
Preventative treatment can help reduce the frequency of migraine attacks, helping children and adolescents to better manage the condition day-to-day. AJOVY is administered once a month and available for in-office or at home use, offering a treatment option that is intended to support adherence and reduce treatment burden for families.
“Migraines are a common yet invisible condition that can severely disrupt daily life for children and adolescents, often leaving them overlooked and misunderstood,” said Chris Fox, Executive Vice President, U.S. Commercial and Innovative Franchise Lead and Head of Global Marketing Business at Teva. “With this FDA approval, AJOVY now offers younger patients a new treatment option, addressing a long-standing gap in care and offering families added support as they navigate the challenges of this condition.”
1 in 10 children and adolescents in the U.S. suffer from migraine, one of the most common and disabling neurological conditions. Despite its widespread prevalence, pediatric migraine is often underrecognized and undertreated, contributing to missed school days, difficulties with schoolwork, and disrupted social activities.
“Pediatric migraine is a complex condition that can significantly impact a child’s daily life, from school performance to emotional well-being,” said Dr. Jennifer McVige, MD, MA, Pediatric Neurologist at the DENT Neurologic Institute. “Having an FDA-approved treatment like AJOVY offers an important option, providing a targeted approach to preventive treatment for episodic migraine that can help reduce the frequency of attacks in younger patients and help clinicians manage this often-overlooked condition.”
Building on its established success in adult patients since its U.S. approval in 2018, this expanded indication strengthens Teva’s commitment to broadening access to neuroscience therapies across age groups.2 AJOVY continues to demonstrate efficacy in addressing the underlying biology of migraine and now provides a treatment pathway for a patient population with historically limited preventive options.
Migraine attacks cause disabling pain, nausea, vomiting and sensitivities to light and sound, resulting in serious effects on the ability to complete daily tasks.5 Migraine can cause significant disability in children and adolescents, leading to absence from school, impaired educational performance and missed social activities.3
AJOVY is indicated for preventive treatment of migraine in adults and episodic migraine in children and adolescent patients aged 6-17 years who weigh 45 kilograms (99 pounds) or more. AJOVY is available as a 225 mg/1.5 mL single dose injection in a pre-filled autoinjector or in a pre-filled syringe. AJOVY can be administered either by a healthcare professional or at home by a patient or caregiver. No starting dose is required to begin treatment.
Powered by WPeMatico
In a major advance for patients with Crohn’s disease, a new study led by researchers at Mount Sinai Health System found that guselkumab, a medication with a mechanism of action that is new to inflammatory bowel disease (IBD) treatment, outperformed an established standard of care in promoting intestinal healing and symptom relief.
These findings from two pivotal phase 3 trials known as GALAXI 2 and 3, published today in The Lancet, provided the basis for the recent Food and Drug Administration approval of guselkumab (brand name Tremfya) for the treatment of moderately to severely active Crohn’s disease.
Crohn’s disease affects roughly 780,000 people in the United States and often requires a lifetime of management. Despite numerous available biologic medications, many patients fail to achieve sustained remission. Guselkumab blocks the interleukin-23 (IL-23) pathway, a key driver of chronic intestinal inflammation.
“Suboptimal disease control despite the availability of biologic therapies remains a prevalent problem among patients with Crohn’s disease,” said Bruce E. Sands, MD, MS, the Dr. Burrill B. Crohn Professor of Medicine at the Icahn School of Medicine at Mount Sinai and senior author on this paper. “The GALAXI trials were especially impactful as they compared two dosing regimens of guselkumab to both placebo and ustekinumab over a 48-week period. Patients receiving guselkumab showed significantly higher rates of endoscopic healing and deep remission, critical indicators linked to fewer disease flares, hospitalizations, and long-term complications.”
Guselkumab is the first biologic shown in identically designed, 48-week, double-blind trials to outperform another leading biologic—ustekinumab, a monoclonal antibody that blocks both IL-12 and IL-23—for key markers of disease remission and gut healing in Crohn’s disease. The design allowed for head-to-head comparisons to both placebo and ustekinumab. The studies enrolled 1,048 patients worldwide and randomly assigned participants to one of four groups:
• guselkumab 200 mg intravenously (IV) at weeks 0/4/8, then guselkumab 100 mg subcutaneously (SC) every eight weeks beginning at week 16
• guselkumab IV at weeks 0/4/8, then guselkumab 200 mg every four weeks beginning at week 12
• ustekinumab ~6 mg/kg IV at week 0, then ustekinumab 90 mg SC every eight weeks beginning at week 8
• placebo
Guselkumab demonstrated statistically significant improvements across multiple endpoints, including endoscopic response and deep remission. Safety outcomes, based on adverse events and laboratory findings, were favorable and consistent with the known profile of guselkumab in approved indications.
Beyond symptom control, the therapy’s corticosteroid-sparing effect further underscores its clinical value, especially for patients seeking alternatives to long-term steroid use.
The GALAXI 2 and 3 trials were sponsored by Johnson & Johnson and enrolled patients with prior biologic treatment failures.
Dr. Sands, the Chief of Mount Sinai’s Dr. Henry D. Janowitz Division of Gastroenterology, is a renowned expert in inflammatory bowel disease. He previously co-authored the pivotal UNITI trial, and was both the lead author and corresponding author on the UNIFI trials. Both of these trials helped establish ustekinumab’s place in IBD care. His latest work builds on that legacy, demonstrating not only the effectiveness of IL-23 inhibition but its potential to redefine front-line treatment.
Powered by WPeMatico

The MRI image shows a brain tumor in an inauspicious location, – and a brain biopsy will entail high risks for the patient, who had consulted us due to double vision. Situations such as this case discussion, cited by way of example, in a multidisciplinary team of cancer medicine experts prompted researchers at Charité – Universitätsmedizin Berlin, together with cooperation partners, to look for new diagnostic procedures. The result is an AI model. The model makes use of specific characteristics in the genetic material of tumors – their epigenetic fingerprint, obtained for example from cerebrospinal fluid, among other things. As the team shows in the journal Nature Cancer*, the new model classifies tumors quickly and very reliably.
Today, far more types of tumors are known than the organs from which they arise. Each tumor has its own characteristics, certain tissue features, growth rates and metabolic peculiarities. Nevertheless, tumor types with similar molecular characteristics can be grouped together. The treatment of the individual disease depends decisively on the type of tumor. New, targeted therapies address certain structures of tumor cells or block their signaling pathways in order to halt the pathological tissue growth. Chemotherapies can be selected according to tumor type and their dosage adjusted accordingly. Particularly in the case of rare tumor types, it may be possible to pursue innovative therapies as part of studies.
“Against the backdrop of increasingly personalized, rapidly developing cancer medicine, precise diagnosis at a certified tumor center is the way forward for successful treatment,” as Prof. Martin E. Kreis, Chief Medical Officer at Charité, stated. While a comprehensive molecular, cellular and functional analysis of a tumor based on tissue samples provides the necessary information, physicians are also confronted with cases in which it is not possible or very risky to extract tissue samples from the tumor. What’s more, even a histological examination alone is not capable of providing as precise a diagnosis as the new AI model.
A method has been established for characterizing brain tumors that is not based on conventional microscopic diagnostics, but instead on modifications of the tumor‘s genetic material, the epigenetic characteristics. They are part of the memory of every cell and determine which parts of the genetic information are read and when. “Hundreds of thousands of epigenetic modifications act as on and off switches for individual gene sections. Their patterns form a unique, unmistakable fingerprint,” explains Dr. Philipp Euskirchen, scientist at the Berlin site of the German Cancer Consortium and at the Institute of Neuropathology at Charité, who heads the recently published study. “In tumor cells, the epigenetic information is altered in a characteristic way. Based on their profiles, we can differentiate between tumors and classify them.” In the case of brain tumors, even a sample of the cerebrospinal fluid is sufficient in some cases, and can be obtained relatively easily – dispensing with surgery altogether.
In order to compare an unknown fingerprint with thousands of known fingerprints of different cancers and assign it to a specific tumor type, machine learning methods, i.e., artificial intelligence, are required given that the data are very extensive and complex. What’s more, different DNA sequencing methods were applied in the past. In addition, epigenetic analyses are usually limited to defined patterns and gene segments that are typical for individual tumor types. “Consequently, our aim was to develop a model that accurately classifies tumors, even if they are only based on parts of the entire tumor epigenome or the profiles were collected by means of different techniques and varying degrees of accuracy,” as bioinformatician Dr. Sören Lukassen, head of the Medical Omics working group at the Berlin Institute of Health at Charité (BIH), stated.
A newly developed AI model goes by the name of crossNN, whose architecture is based on a simple neural network. The model was trained with a large number of reference tumors and subsequently tested on more than 5,000 tumors. “Our model allows a very precise diagnosis of brain tumors in 99.1 percent of all cases and is more accurate than the AI solutions at work to date,” as Philipp Euskirchen related. “In addition, we were able to train an AI model in the same way that can differentiate between over 170 tumor types from all organs, while achieving accuracy of 97.8 percent. This means that it can be used for cancers of all organs, in addition to the relatively rare brain tumors.” The decisive factor for future approvals in clinical application is that the models are fully explainable, i.e., it must be possible to understand how the decisions are arrived at.
The molecular fingerprint that the AI model receives for determination can stem from a tissue sample or from body fluids. In the case of specific brain tumors, the Department of Neuropathology at Charité already offers non-invasive diagnostics based on cerebrospinal fluid, known as liquid biopsy. This allows a diagnosis to be made without a stressful operation, also in difficult situations. The patient who consulted us with double vision was one of the beneficiaries. “We examined the cerebrospinal fluid using nanopore sequencing, a novel, very fast and efficient form of genetic analysis. The classification by our models revealed that it was a lymphoma of the central nervous system, enabling us to promptly initiate appropriate chemotherapy,” Philipp Euskirchen explains.
The accuracy of the methodology even took the researchers by surprise. “Although the architecture of our AI model is far more simple than previous approaches and therefore remains explainable, it delivers more precise predictions and therefore greater diagnostic certainty,” says Sören Lukassen. Together with the German Cancer Consortium (DKTK), the research team is therefore planning clinical trials with crossNN at all eight DKTK locations in Germany. In addition, intraoperative use is also to be tested. The aim is to transfer the precise and comparatively inexpensive tumor determination based on DNA samples to routine care.
Reference:
Yuan, D., Jugas, R., Pokorna, P. et al. crossNN is an explainable framework for cross-platform DNA methylation-based classification of tumors. Nat Cancer 6, 1283–1294 (2025). https://doi.org/10.1038/s43018-025-00976-5.
Powered by WPeMatico
