FDA Approves Delgocitinib Cream for Chronic Hand Eczema
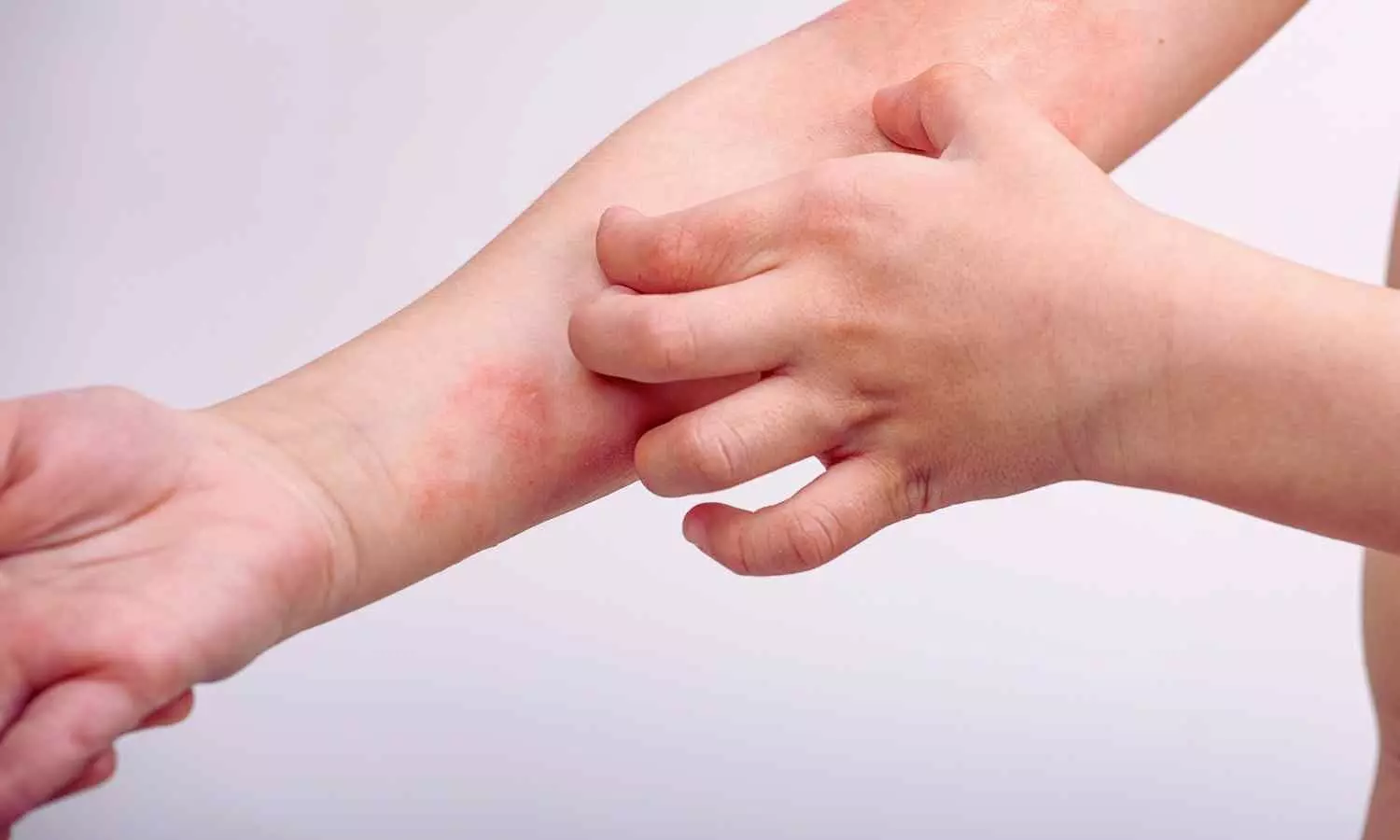
The FDA has approved delgocitinib cream as a topical treatment for moderate-to-severe chronic hand eczema (CHE) in adults, according to the manufacturer’s announcement on July 23, 2025.
ANZUPGO is an innovative steroid-free, topical pan-Janus kinase (JAK) inhibitor for adults with CHE.1 ANZUPGO inhibits the JAK-STAT pathway, specifically blocking the activity of JAK1, JAK2, JAK3, and tyrosine kinase 2 (TYK2), and suppresses the various inflammatory responses that play a key role in the onset and subsequent flares of CHE.
The FDA approval of ANZUPGO marks a significant milestone in LEO Pharma’s strategy to expand its presence in the U.S. market and deliver purposeful innovation in skin health. In preparation for bringing ANZUPGO to the U.S. patients, LEO Pharma has significantly upscaled its operations across key functions – including a 50% increase in the sales force.
“ANZUPGO is a good example of how we transform a real need in the market into medicines that can help make a difference for people living with serious skin diseases such as CHE,” said Christophe Bourdon, CEO, LEO Pharma. “After successfully launching ANZUPGO in several countries, we’re proud to now bring this innovation to adult patients with moderate-to-severe CHE in the United States. The approval of ANZUPGO reinforces our commitment to investing in difficult-to-treat skin conditions to deliver new treatments to patients where the need is greatest. We’re truly grateful to the patients and physicians who participated in our studies and helped make this approval possible.”
CHE is a highly debilitating inflammatory skin disease that affects approximately one in ten adults worldwide, causing itchy, painful, blistered, or swollen skin that can interfere with daily activities. The FDA approval of ANZUPGO provides adults in the U.S. living with moderate-to-severe CHE with the first and only treatment option specifically approved for this skin disease, just as it will be the first and only topical pan-JAK-inhibitor on the U.S. market.
“Chronic hand eczema can be a very difficult disease for adults to manage, especially given the lack of treatment options in the U.S. until now,” said Robert Spurr, EVP and President, North America, LEO Pharma. “As the first and only FDA-approved treatment specifically for CHE in the U.S., ANZUPGO further establishes our company’s real commitment to bringing treatments to market that address unmet needs in medical dermatology.”
The FDA approval is the latest regulatory milestone for ANZUPGO, following the European Commission (EC) approval in 2024 and several launches internationally, including Germany, Switzerland, the United Kingdom and the United Arab Emirates.
About ANZUPGO® (delgocitinib) Cream
ANZUPGO® (delgocitinib) cream is currently FDA approved in the U.S. as the first and only treatment for chronic hand eczema (CHE). ANZUPGO is also approved in the European Union, United Kingdom, Switzerland and the United Arab Emirates for the treatment of moderate-to-severe chronic hand eczema (CHE) in adults for whom topical corticosteroids are inadequate or not advisable. ANZUPGO cream is also under investigation in other markets. Use of ANZUPGO in combination with other JAK inhibitors or potent immunosuppressants is not recommended by the U.S. FDA.
ANZUPGO cream is a topical pan-Janus kinase (JAK) inhibitor for the treatment of moderate-to-severe CHE in adults. It inhibits the activation of JAK-STAT signaling, which plays a key role in the pathogenesis of CHE.
In 2014, LEO Pharma A/S and Japan Tobacco Inc. (JT) entered into a license agreement in which LEO Pharma gained exclusive rights to develop and commercialize delgocitinib for topical use in dermatological indications worldwide, excluding Japan, where JT retains rights.
About Chronic Hand Eczema
Chronic hand eczema (CHE) is defined as hand eczema (HE) that lasts for three or more months or relapses twice or more within a year. HE is one of the most common skin disorders of the hands and in a substantial number of patients, it can develop into a chronic condition. CHE affects approximately one in ten adults worldwide. It is a fluctuating disorder characterized by itch and pain, and patients may experience signs such as erythema, scaling, lichenification, hyperkeratosis, vesicles, edema, and fissures on hands and wrists.6 The pathophysiology is characterized by skin barrier dysfunction, inflammation of the skin, and alterations of the skin microbiome.
CHE has been shown to cause psychological and functional burdens that impact patient quality of life, with approximately 70% of individuals who live with severe CHE admitting to problems in performing everyday activities. Furthermore, careers and earning potential have also been shown to be impacted by the burden of living with CHE.
Powered by WPeMatico