Inside Coldrif Scandal: Tamil Nadu Plant Used Paint-Grade Chemicals, Violated 300+ Drug Norms

Chennai: Officials from the Tamil Nadu Drugs Control Department uncovered horrific conditions at the Sresan Pharmaceuticals unit in Kancheepuram, where the now-banned Coldrif cough syrup was manufactured. The raid exposed open vats of boiling chemicals, leaking rusted pipes, and workers handling toxic substances without masks or gloves, a scene investigators described as a “factory of death.”
Acoording to a recent media report in the MoneyControl, batch SR-13 of Coldrif Syrup, linked to multiple child deaths in Madhya Pradesh’s Chhindwara district, was produced in May 2025 and carried an expiry date of April 2027. For months, the syrup had been in circulation before being flagged for contamination. During the inspection conducted on October 1 and 2, authorities found that the company was using industrial-grade chemicals meant for paints and adhesives instead of pharmaceutical-grade raw materials. Purchases were made from two Chennai-based dealers — Sunrise Biotech and Pandia Chemicals — through cash and Google Pay to avoid leaving financial trails.
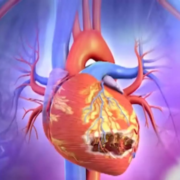
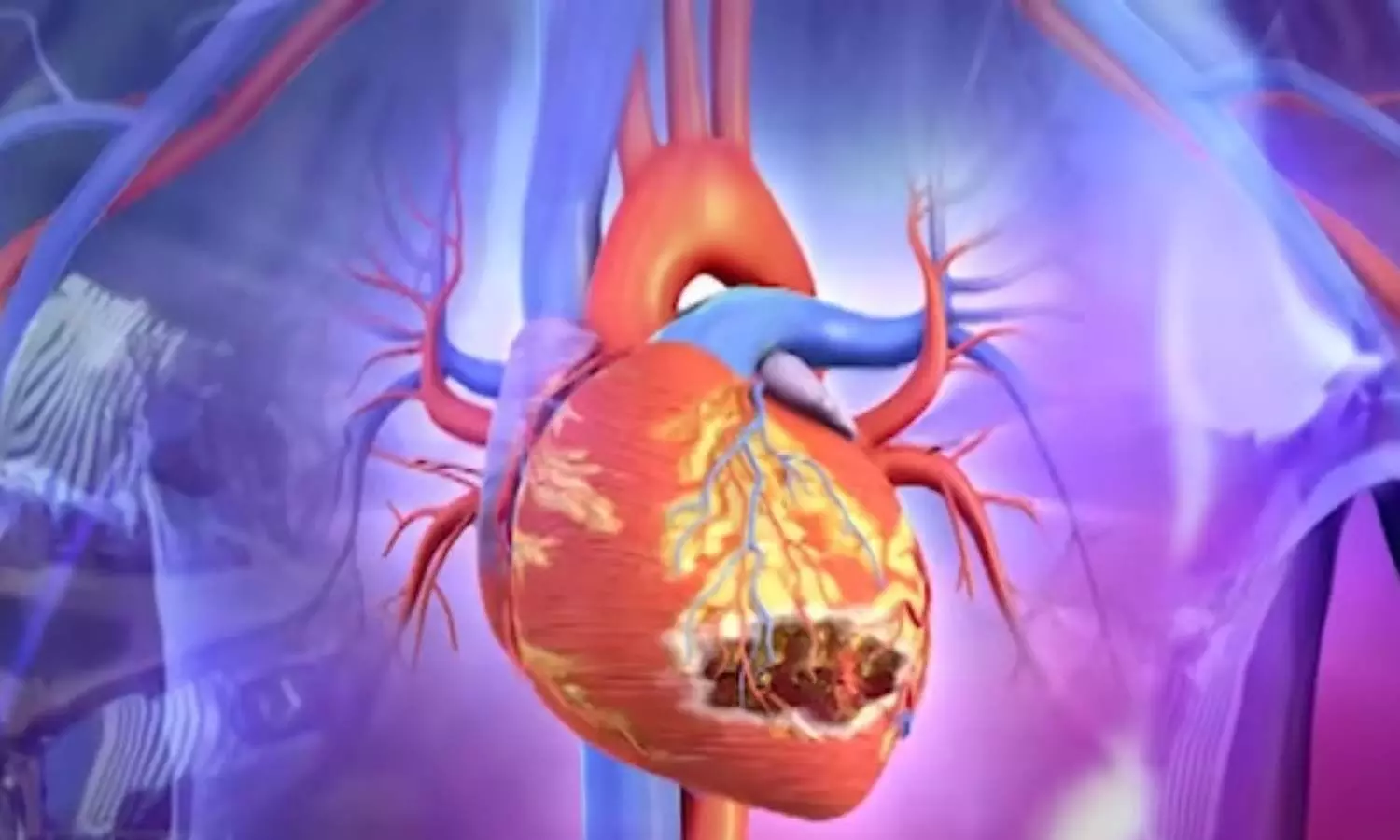
The probe revealed that the propylene glycol used in the syrup contained diethylene glycol (DEG), a highly toxic compound commonly found in brake fluid and lubricants. Laboratory analysis later confirmed that 48.6% of the offending batch consisted of DEG — nearly 500 times the permissible limit. Post-mortem samples from affected children confirmed kidney failure caused by DEG poisoning.
Inspectors catalogued 39 critical and 325 major violations of the Drugs and Cosmetics Act, 1940, at the Kancheepuram facility. The lapses included the absence of qualified chemists, unhygienic conditions, use of untreated water, missing quality control records, open drains, and lack of pest control. Investigators said the facility lacked any semblance of Good Manufacturing Practices (GMP), with safety protocols ignored and documentation falsified.
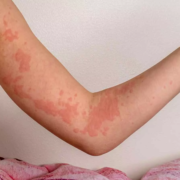
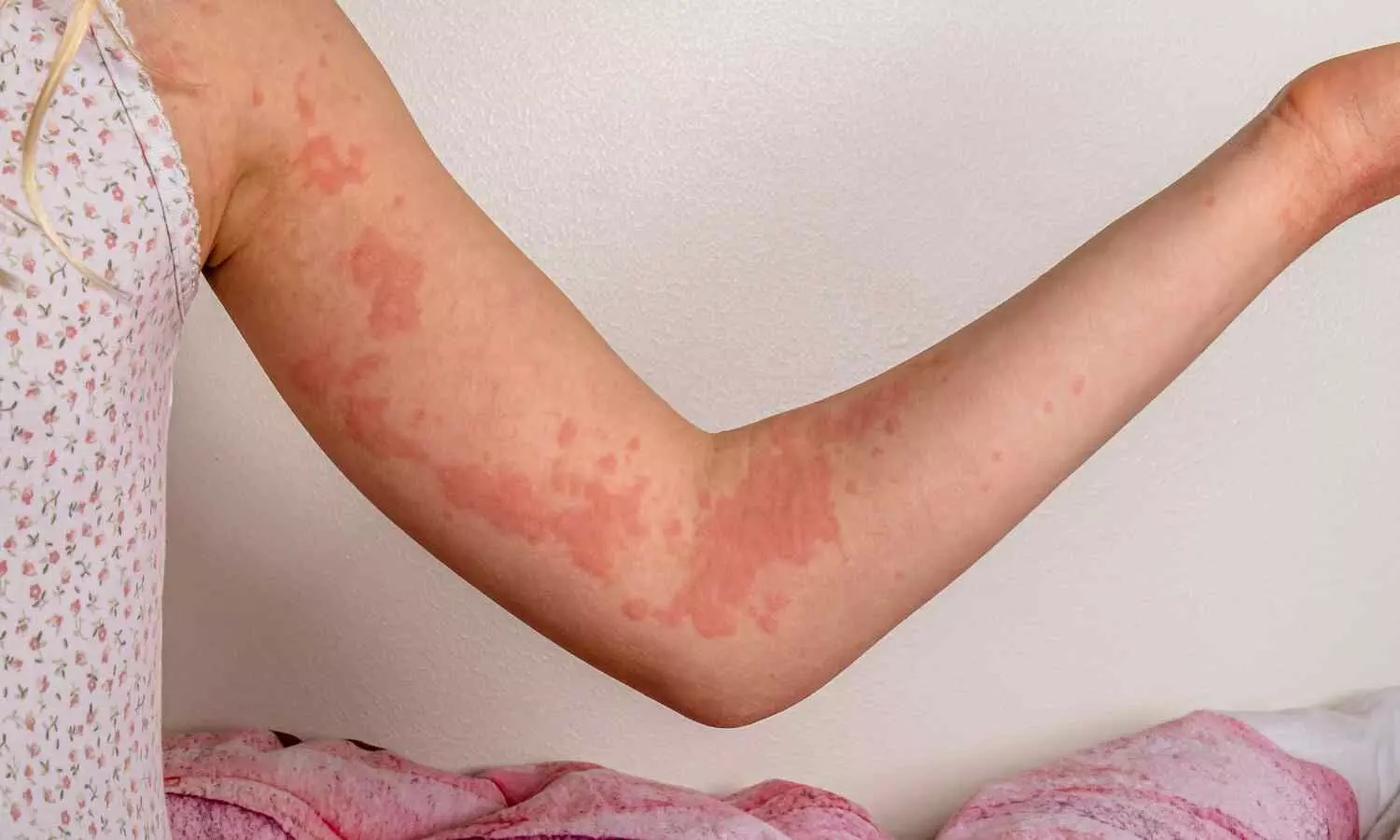
Batch SR-13 of Coldrif was distributed in Tamil Nadu, Odisha, Puducherry, and Madhya Pradesh. The children who consumed it developed fever and cough before suffering acute kidney failure. Reports indicate that at least 15 children have died so far. Following the findings, Tamil Nadu’s Drugs Control Department suspended Sresan Pharmaceuticals’ license and issued a show-cause notice. Meanwhile, in Madhya Pradesh, several drug inspectors and a deputy director were suspended, the state’s Drug Controller was transferred, and a local doctor was arrested for alleged negligence, reports Moneycontrol.
The revelations have sparked nationwide outrage and renewed scrutiny of India’s pharmaceutical manufacturing practices, exposing severe lapses in raw material procurement, quality assurance, and post-market surveillance.
Powered by WPeMatico