Jaipur: The Comptroller and Auditor General of India‘s audit of Public Health Infrastructure and Management of Health Services in Rajasthan revealed that neither the composition nor the functioning of the Rajasthan Medical Council was in accordance with the requirements of the Rajasthan Medical Act. In this regard, the CAG report called for the State Government’s action to monitor the functioning of the State Medical Council to ensure effective regulation by the body.
The Performance Audit, covering the period between 2016-2022, assessed the healthcare services provided in the State of Rajasthan.
It examined whether the Government of Rajasthan took adequate measures to ensure adequate availability of infrastructure and healthcare services, drugs, medicines, equipment, and human resources to beneficiaries.
Here are the key takeaways:
(1) Shortage of Doctors, and Specialists:
In the report, the Audit observed that the State was reeling under a shortage of doctors, specialists, nurses, and paramedics in the primary, secondary, and tertiary healthcare institutes.
“The State Government should address the shortage of health care professionals in a time-bound manner by adopting a clearly defined recruitment strategy as well as ensuring their equitable distribution across the State,” mentioned the Audit.
(2) Essential Minimum Assured Services Deficient in IPD of Government Medical Institutes:
The Audit observed that essential minimum assured services like General Surgery, Paediatrics, Orthopaedics, and Accident and Trauma Wards were deficient in IPD of the Government Medical Institutions (GMIs). Further, it also noted that there were deficiencies in services such as Emergency, Intensive Care Units, Blood Banks of GMIs.
In this regard, the CAG report opined that the State should ensure availability of the required Minimum Assured Services, like General Surgery, Paediatrics, Orthopaedics, Emergency, Intensive Care Unit, Blood Banks, etc. as per IPHS in the GMIs of the State.
(3) Non-availability of Radiology, Pathology, Dietary, Ambulance and Mortuary Services:
The CAG report took note of the non-availability of adequate Radiology, Pathology, Dietary, Ambulance, and Mortuary services as per IPHS in the test-checked GMIs.
The Radiology service is available in 12 DHs out of 34 DHs, Pathology services are partially available in all 34 DHs, Dietary services are available in 18 out of 34 DHs, Ambulance services are available in 7 out of 34 DHs, and Mortuary service is available only in one DH our of the 34 DHs, revealed the audit.
Addressing the issue, the audit opined that the State Government should make efforts to provide adequate services such as Radiology, Pathology, Dietary, Ambulance and Mortuary Services as per IPHS in the GMIs of the State.
(4) Shortfall of essential drugs:
As per the CAG report, the State had a shortfall of availability of essential drugs in test-checked GMIs and district drug warehouses. For this, the audit advised the State to take necessary steps to ensure sufficient availability of essential drugs in the hospitals and drug warehouses.
Audit observed that medicines issued to medical units were less than the quantity demanded and that there were delays in finalizing rate contracts. It was also observed that purchase orders were placed when the buffer stock was much less than the prescribed buffer level.
In this regard, the CAG report advised the State to specify the re-order point at which purchase orders are placed and put in place a mechanism of system-generated purchase orders for maintenance of prescribed buffer stocks.
(5) Delay in Test Reports:
The audit also highlighted that there were delays in the receipt of quality test reports from the laboratories which resulted in the delay in issuing medicines by the warehouses to the medical units, contributing to the non-availability of medicines at the medical units. Addressing this, the audit asked the State to ensure timely testing of drug samples and receipt of quality test reports on time.
Taking note of the fact that test-checked GMIs purchased drugs locally without quality test reports, the audit asked the State to ensure issue of drugs to GMIs only after receipt of quality test reports.
(6) Inadequate equipment:
The CAG report noted that equipment required as per Indian Public Health Standards (IPHS) in various categories was not available in the test-checked GMIs and therefore, the audit asked the State to ensure availability of essential equipment in the GMIs as per IPHS, as well as their periodic maintenance and calibration.
(7) Inadequate Health Facilities in Rural areas:
While assessing the healthcare infrastructure across the desert, tribal, and plain areas of the State, the audit noted that there were shortage of healthcare facilities as the State did not have a concrete plan to address the shortage and prioritise areas with shortfall for setting up of healthcare infrastructure.
Accordingly, the audit advised the State to ensure availability of healthcare institutes as per the Indian Public Health Standards in the State and their equitable distribution to make healthcare accessible for all.
(8) Deficiencies in Basic Amenities:
The audit also noticed deficiencies in the provision of basic amenities like separate toilet facilities for male and female, electricity, and potable drinking water in the Government Medical Institutions (GMIs) of the State and asked the Government to ensure availability of basic amenities like separate toilet facilities, electricity and potable drinking water in all the Government Medical Institutions of the State.
(9) Expenses in Healthcare Sector:
Noting that the capital expenditure constituted only 6.67 per cent of the total expenditure on healthcare, the Audit asked the State Government to make efforts to augment the capital expenditure in healthcare sector.
The percentage of out-of-pocket expenditure to total health expenditure decreased in Rajasthan during the period 2016-20. However, it constituted 47.40 per cent of total healthcare expenditure during 2019-20, indicating low financial protection available to households towards healthcare payments in the State, it noted.
Accordingly, it directed the State Government to take necessary steps to increase the budgetary allocation for healthcare sector both as a percentage of State Budget as well as Gross State Domestic Product in line with the National Health Policy.
(10) Non Functioning Health and Wellness Centres:
The audit asked the State to ensure proper functioning of Health and Wellness Centres as envisaged under the Ayushman Bharat-Health Wellness Centre as it noted that there was non-availability of required tests, non-conduct of Yoga sessions, and inadequate screening of Non-Communicable Diseases in adults in the Health and Wellness Centres established under Ayushman Bharat scheme in the State.
(11) Delay in Renewing Drug Manufacturing Licenses:
In the CAG report, the delays in issuing and renewing of drug manufacturing licenses were highlighted and it also noted that the regular annual inspections were not carried out in any of the test-checked manufacturing units.
Addressing the issue, the State Government was advised to ensure that issue and renewal of manufacturing licenses are done in a time-bound manner and annual inspections are conducted regularly.
(12) Delayed Action Against Firms Manufacturing Below Quality Drugs:
The Audit also noted that the action against firms manufacturing Not of Standard Quality drugs was significantly delayed. Accordingly, it opined that the State Government should fix responsibility of officers concerned for failure to ensure timely and appropriate action against the manufacturers of Not of Standard Quality Drugs.
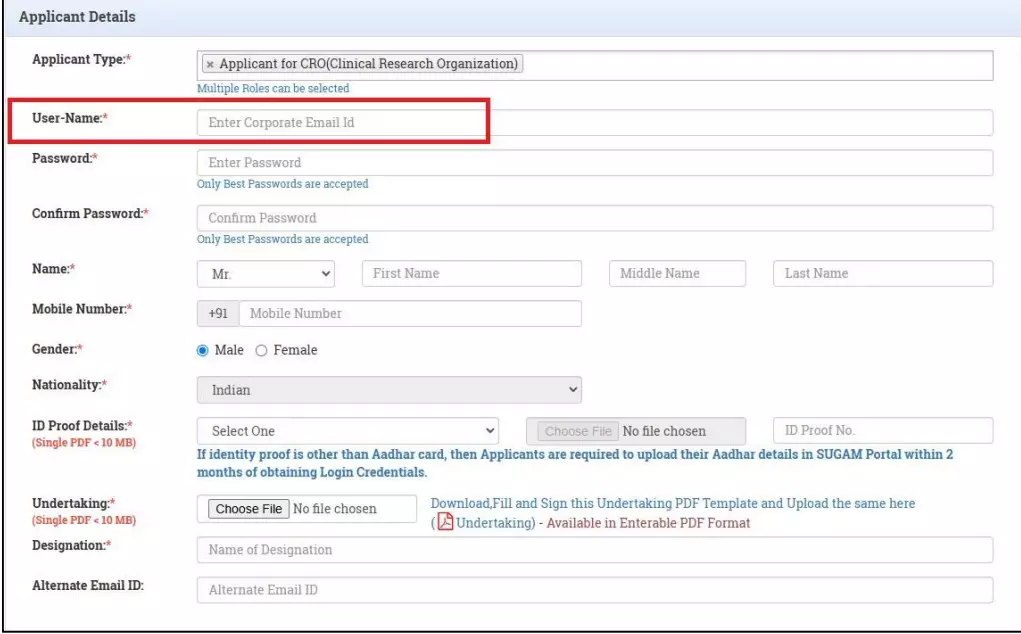
(13) Issues in Rajasthan Medical Council and State Pharmacy Council:
Regarding the Rajasthan Medical Council, the audit observed that neither the composition nor the functioning of the Council was in accordance with the requirements of Rajasthan Medical Act. It also noted that the State Pharmacy Council had not appointed Pharmacy Inspectors in the State.
The audit opined that the State Government should monitor the functioning of the State Medical Council and State Pharmacy Council to ensure effective regulation by these bodies.
Finally, noting that the State of Rajasthan had not formulated any vision/roadmap/strategy for achievement of Sustainable Development Goals (SDGs), it asked the State Government to expedite the alignment of SDGs with the State Government Vision 2030.
Also Read:Delhi Healthcare- Here are 7 shortcomings listed by CAG report