Virtual reality could help stroke survivors regain movement
Powered by WPeMatico
Powered by WPeMatico
Powered by WPeMatico
Powered by WPeMatico
Powered by WPeMatico
Powered by WPeMatico
Powered by WPeMatico

A new study using one of the world’s largest cancer registries shows that patient outcomes can be significantly impacted depending on when colorectal cancer (CRC) is diagnosed related to other cancers. The results are published in the Journal of the American College of Surgeons (JACS).
The Surveillance, Epidemiology, and End Results (SEER) Program is a cancer database established by the National Cancer Institute. Researchers studied data from 2000-2020 and defined three different groups:
Key Findings:
“We expected isolated CRC patients to fare best, but patients with CRC diagnosed first, followed by another cancer had the best survival rates. This was surprising; we hypothesized patients with only a CRC diagnosis would do best,” said first author Anjelli Wignakumar, MBBS, BSc (Hons), a clinical research fellow at the Department of Colorectal Surgery, Ellen Leifer Shulman & Steven Shulman Digestive Disease Center at Cleveland Clinic Florida.
There are multiple potential reasons why Group B patients, with multiple cancers, would fare better than Group A, who only had CRC. The first is the increased surveillance and interaction with the medical system that comes with cancer diagnosis. Increased monitoring leads to cancers being caught earlier. Prior cancer treatment may prime the immune system to fight subsequent cancers. Healthier habits post-diagnosis could also improve outcomes.
Another difference was that “Group A patients were younger, presented more aggressively (higher liver metastases), and were less likely to receive surgery — possibly because their disease was further along,” said Dr. Wignakumar.
For providers, the researchers emphasize that patients with a prior history of non-CRC cancer may require more intensive CRC screening. “Group C (CRC as the second cancer) had the worst outcomes. Clinicians must treat these as high-risk cases and consider aggressive therapy,” said Sameh H. Emile MBBCh, MSc, MD, FACS, co-author and a project scientist at the Department of Colorectal Surgery, Ellen Leifer Shulman & Steven Shulman Digestive Disease Center, Cleveland Clinic Florida.
For patients: “Surviving CRC doesn’t make you immune to other cancers, but the next one could have better outcomes,” emphasized Steven D. Wexner, MD, PhD (Hon), FACS, senior author, and director of the Ellen Leifer Shulman & Steven Shulman Digestive Disease Center at Cleveland Clinic Florida. “Follow all recommended screenings — catching the next cancer early saves lives.”
Reference:
Wignakumar, Anjelli, Does the Sequence of Colorectal Cancer Diagnosis Matter for Patients with Multiple Primary Cancers? A Surveillance, Epidemiology, and End Results Database Cohort Study, Journal of the American College of Surgeons, DOI: 10.1097/XCS.0000000000001413.
Powered by WPeMatico

A new kind of smart contact lens is poised to revolutionize how we monitor eye health. Designed to work even when the eyes are closed, this stretchable lens combines pressure and movement sensing in one compact, wireless system. It offers a noninvasive, high-resolution way to track key ocular signals like intraocular pressure (IOP) and eye movement (EM)-factors critical for managing chronic conditions such as glaucoma. Successfully tested in both animal and human models, the lens provides accurate, real-time data to external devices without disrupting vision or comfort. This innovation could mark a turning point in how we detect and manage eye diseases.
Vision disorders impact over a billion people worldwide, with glaucoma being one of the most insidious due to its slow, symptomless progression. Key biomarkers like elevated IOP and irregular eye movement (EM) often go unnoticed, especially during sleep, when traditional open-eye instruments fall short. Nocturnal like intraocular pressure (IOP) spikes and rapid eye movement (REM)-related EM events are particularly important for diagnosing and preventing damage. Smart contact lenses have emerged as a promising solution, but most are limited to open-eye use and struggle to capture dual-modal data with precision. Because of these limitations, there’s a growing need for wearable devices that can deliver continuous, closed-eye monitoring with both accuracy and comfort.
Now, researchers from the University of Electronic Science and Technology of China have developed a next-generation smart contact lens that does exactly that. Published (DOI: 10.1038/s41378-025-00946-y) in Microsystems & Nanoengineering in May 2025, the study unveils a soft, stretchable bimodal contact lens (BCL) capable of simultaneously monitoring IOP and EM, even with the eyelids shut. By integrating capacitive and magnetic sensors into a single, wirelessly connected platform, the device enables round-the-clock tracking of eye health, offering a new pathway toward personalized, proactive care.
The BCL combines five layers of carefully engineered materials, including serpentine copper coils for pressure sensing and a neodymium-infused magnetic film for movement detection. This flexible structure allows the lens to contour naturally to the eye, avoiding discomfort and preserving vision. In rabbit models, the lens demonstrated high sensitivity to IOP fluctuations, with resolution as fine as 1 mmHg and superior signal stability even when eyes were closed. EM detection achieved over 97% accuracy in both lab models and human trials, thanks to an integrated glasses-mounted Tesla meter array. Data from the lens are wirelessly relayed to mobile devices, supporting real-time feedback. Importantly, biocompatibility testing confirmed the device is safe for extended wear, with no signs of inflammation or visual disruption. Together, these features present a rare combination of precision, comfort, and practicality for clinical and everyday use.
“This technology bridges a long-standing gap in ophthalmic care,” says Dr. Guang Yao, co-lead author of the study. “The ability to monitor both IOP and EM continuously—even when the eyes are closed-offers a more complete picture of eye health. It enables early intervention and more accurate tracking of disease progression, particularly for glaucoma patients. And because it’s wireless and wearable, it can be used comfortably at home, not just in clinics.”
Beyond glaucoma, this dual-sensing lens could prove useful in monitoring attention-related disorders, neurodegenerative conditions, and even sleep quality through EM patterns. Its wireless integration with mobile devices means patients can share data with physicians remotely, supporting telemedicine and reducing clinical visits. Future versions may incorporate drug delivery features, turning the lens into a closed-loop diagnostic and therapeutic system. With its customizable design and proven safety, the BCL platform opens up new possibilities for smart medical wearables in personalized healthcare.
Reference:
Gan, X., Yao, G., Li, C. et al. Closed-eye intraocular pressure and eye movement monitoring via a stretchable bimodal contact lens. Microsyst Nanoeng 11, 83 (2025). https://doi.org/10.1038/s41378-025-00946-y
Powered by WPeMatico
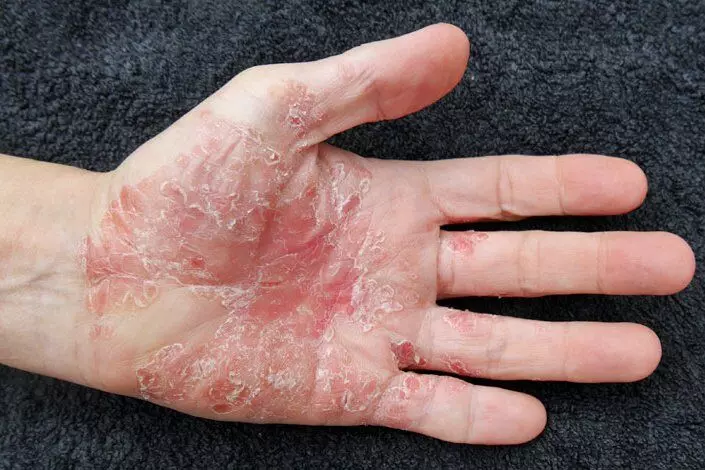
Researchers have found in a recent systematic review and meta-analysis that psoriasis is an independent risk factor for metabolic syndrome (MetS), with a pooled prevalence of 26.49% among psoriasis patients. The risk is notably higher in those with more severe disease, as indicated by elevated PASI scores, indicating the role of disease activity in mediating MetS risk.
A study was done to explore the association between psoriasis and metabolic syndrome (MetS) and analyze the impact of disease activity on the risk of MetS occurrence. This systematic review and meta-analysis used computer searches to search for relevant literature on psoriasis and MetS in databases including China National Knowledge Infrastructure (CNKI), PubMed, Web of Science, Cochrane Central Register of Controlled Trials (CENTRAL) and Embase. The search period was from the establishment of the database to February 8, 2025. Inclusion in case-control, cohort studies and cross-sectional, with language restrictions in Chinese and English during retrieval. After independent screening of literature, extraction of data, and evaluation of risk bias for inclusion in the study by two evaluators, meta-analysis and subgroup analysis were conducted using Stata17.0 software. Result: A total of 12 studies were analyzed, encompassing 9,641 patients with psoriasis and 2,554 patients suffering from MetS alongside psoriasis. The incidence of metabolic syndrome in psoriasis patients was analyzed and the combined effect size was 26.49% [95% CI (25.61, 27.39%)]. Results from the meta-analysis indicated that, in comparison to the control group, psoriasis patients demonstrated a heightened risk of developing MetS [OR = 1.27, 95% CI (1.21-1.33), p < 0.001]. Subgroup analysis revealed that patients with severe psoriasis (PASI≥10) had a significantly increased risk of developing metabolic syndrome [OR = 2.25 95% CI (1.27, 3.99), p < 0.001], indicating that greater disease activity is associated with an elevated likelihood of MetS occurrence. Psoriasis is positively correlated with MetS risk, and increased disease activity further increases the risk. It is necessary to strengthen screening for metabolic abnormalities and multidisciplinary management.
Reference:
Li Z, Gu Z, Xiang J, Zhang X. The incidence of metabolic syndrome in psoriasis patients and its correlation with disease activity: a systematic review and meta-analysis. Front Med (Lausanne). 2025 May 2;12:1593003. doi: 10.3389/fmed.2025.1593003. PMID: 40385577; PMCID: PMC12081259.
Keywords:
Psoriasis, Significantly, Increases, Risk, Metabolic, Syndrome, Especially, among, those, Severe, Disease, Study, Li Z, Gu Z, Xiang J, Zhang X, disease activity; incidence; meta; metabolic syndrome; psoriasis.
Powered by WPeMatico

The US Food and Drug Administration has approved lenacapavir (Yeztugo), the first twice-yearly injectable HIV-1 prevention option. As a capsid inhibitor, it offers a significant advancement in PrEP by reducing the burden of daily or bimonthly dosing for adults and adolescents weighing at least 35 kg.
“This is a historic day in the decades-long fight against HIV. Yeztugo is one of the most important scientific breakthroughs of our time and offers a very real opportunity to help end the HIV epidemic,” said Daniel O’Day, Chairman and Chief Executive Officer of Gilead Sciences. “This is a medicine that only needs to be given twice a year and has shown remarkable outcomes in clinical studies, which means it could transform HIV prevention. Gilead scientists have made it their life’s work to end HIV and now, with the FDA approval of Yeztugo and in collaboration with our many partners, we can help to make that goal a reality.”
The first PrEP medication, which was also developed by Gilead, was approved in the U.S. in 2012. However, data from the Centers for Disease Control and Prevention (CDC) show that, in 2022 (the most recent year with available data), only about 1 in 3 (36%) people in the U.S. who met the CDC’s eligibility criteria for PrEP were prescribed a form of PrEP. CDC data show that all populations in the U.S. are not yet using PrEP at rates that could end transmission of the virus at the population level, with particular gaps for women, Black/African American and Hispanic/Latino people, and people in the U.S. South. Data also show that barriers including adherence challenges, stigma and low awareness of existing PrEP options—by both healthcare providers and consumers-contribute to this low uptake of PrEP across multiple populations. The potential impact of this limited uptake, adherence and access is underscored by the fact that, in 2023,more than 100 people were diagnosed with HIV every day in the U.S.
“Yeztugo could be the transformative PrEP option we’ve been waiting for-offering the potential to boost PrEP uptake and persistence and adding a powerful new tool in our mission to end the HIV epidemic,” said Carlos del Rio, MD, Distinguished Professor of Medicine in the Division of Infectious Diseases at Emory University School of Medicine and Co-Director of the Emory Center for AIDS Research in Atlanta. “A twice-yearly injection could greatly address key barriers like adherence and stigma, which individuals on more frequent PrEP dosing regimens, especially daily oral PrEP, can face. We also know that, in research, many people who need or want PrEP preferred less frequent dosing.”
FDA approval of Yeztugo is supported by high efficacy and demonstrated safety data in two clinical trials
The FDA approval of Gilead’s New Drug Applications (NDAs) for Yeztugo was supported by data from the Phase 3 PURPOSE 1 and PURPOSE 2 trials conducted by Gilead. In the PURPOSE 1 trial , data at the primary analysis showed twice-yearly subcutaneous Yeztugo demonstrated zero HIV infections among 2,134 participants in the Yeztugo group, 100% reduction in HIV infections and superiority of prevention of HIV infections when compared with once-daily oral Truvada® (emtricitabine 200mg and tenofovir disoproxil fumarate 300mg; F/TDF) in cisgender women in sub-Saharan Africa. In the PURPOSE 2 trial , there were two HIV infections among 2,179 participants in the twice-yearly subcutaneous Yeztugo group, demonstrating 99.9% of participants in the Yeztugo group did not acquire HIV infection and superiority of prevention of HIV infections when compared with once-daily oral Truvada among a broad and geographically diverse range of cisgender men and gender-diverse people. In both trials, Yeztugo also demonstrated superiority of prevention of HIV infections when compared with background HIV incidence (bHIV) and was generally well-tolerated, with no significant or new safety concerns identified. Data from both trials were published in The New England Journal of Medicine and, based in part on the trial results, in December 2024 the journal Science named lenacapavir its 2024 “Breakthrough of the Year.”
Yeztugo received FDA approval under Priority Review. Additionally, in October 2024, Yeztugo was granted Breakthrough Therapy Designation, which is intended to expedite the development and review of new drugs that may demonstrate substantial improvement over available therapy.
Gilead’s U.S. access strategy for Yeztugo is designed to enable broad uptake and availability for individuals with and without insurance coverage
In the U.S., Gilead is working closely with insurers, healthcare systems and other payers with the goal of ensuring broad insurance coverage for Yeztugo. Additionally, for eligible commercially insured individuals with commercial insurance, Gilead’s Advancing Access® Co-Pay Savings Program will reduce out-of-pocket costs to as little as zero dollars.
Gilead is also committed to helping to ensure that people without insurance in the U.S. will be able to benefit from Yeztugo. For those who are eligible, Gilead’s Advancing Access medication assistance program will provide Yeztugo free of charge.
Lenacapavir is approved in multiple countries for the treatment of multi-drug-resistant HIV in adults, in combination with other antiretrovirals. Lenacapavir is also approved in the United States to reduce the risk of sexually acquired HIV in adults and adolescents weighing at least 35kg who are at risk of HIV acquisition.
The multi-stage mechanism of action of lenacapavir is distinguishable from other currently approved classes of antiviral agents. While most antivirals act on just one stage of viral replication, lenacapavir is designed to inhibit HIV at multiple stages of its lifecycle and has no known cross resistance exhibited in vitro to other existing drug classes.
Lenacapavir is being evaluated as a long-acting option in multiple ongoing and planned early and late-stage clinical studies in Gilead’s HIV prevention and treatment research program. Lenacapavir is being developed as a foundation for potential future HIV therapies with the goal of offering both long-acting oral and injectable options with several dosing frequencies, in combination or as a mono agent, that help address individual needs and preferences of people and communities affected by HIV. The journal Science named lenacapavir its 2024 “Breakthrough of the Year.”
Powered by WPeMatico
