Guideline on platelet and plasma transfusion released by American College of Chest Physicians
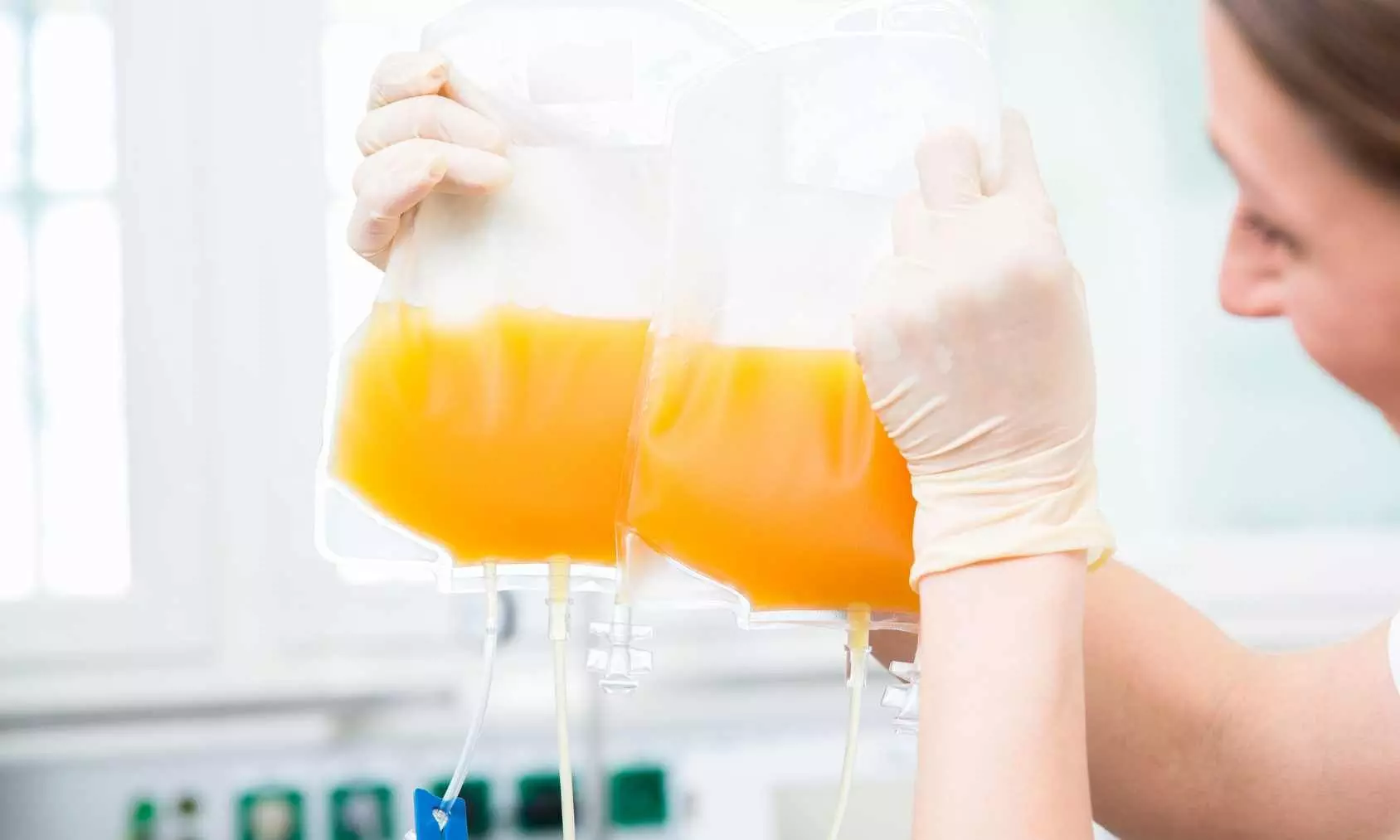
The American College of Chest Physicians® (CHEST) recently released a new clinical guideline on the transfusion of fresh frozen plasma (FFP) andplatelets in critically ill adults. Published in the journal CHEST®, the guideline contains seven evidence-based recommendations to provide a framework for clinicians to implement in their own facilities.
“In practice, we see all too often [that] prophylactic transfusion of platelets or fresh frozen plasma is done unnecessarily,” says Angel Coz Yataco, MD, FCCP, lead author on the guideline. “Transfusion is rarely needed prior to bedside procedures, with lumbar puncture being a possible exception. Following the new guideline, we can drastically reduce the use of FFP.”
The panel of experts developed seven Population, Intervention, Comparator, and Outcome questions addressing platelet and FFP transfusions in critically ill patients and performed a comprehensive evidence review. The panel applied the Grading of Recommendations, Assessment, Development, and Evaluations approach to assess the certainty of evidence and to formulate and grade recommendations.
“In the United States, 20% of platelets and FFP are transfused to critically ill patients, totaling 2.2 million units of each annually,” says Dr. Coz Yataco. “They are scarce resources with variable costs and access globally. If implemented on a large scale, this guideline provides the framework to decrease the use [to] approximately half a million less units of platelets and FFP transfused annually in the United States alone.”
The panel formulated seven conditional recommendations. In addition to four procedure-specific recommendations, the guideline recommends:
• In stable non-bleeding critically ill patients with thrombocytopenia and without high risk of spontaneous bleeding, we suggest transfusing platelets if platelet counts fall below 10 × 109/L (Conditional recommendation, very low certainty of evidence).
• In stable non-bleeding critically ill patients with thrombocytopenia who are considered at high risk of spontaneous bleeding, we suggest transfusing platelets if platelet counts fall below 30-50 × 109/L (Conditional recommendation, very low certainty of evidence).
• In critically ill patients with thrombocytopenia and serious active bleeding, we suggest transfusing platelets if platelet counts fall below 50 × 109/L (Conditional recommendation, very low certainty of evidence).
The entire list of recommendations included in the new guideline are as follows-
Summary of Recommendations
The recommendations in this document apply to critically ill patients, excluding trauma and neuro-critical care populations. These recommendations should be implemented in a hierarchal fashion. Recommendations 1-3 should be applied first to critically ill patients with thrombocytopenia, while recommendations 4-7 provide additional guidance for specific situations involving invasive procedures.
1. In stable non-bleeding critically ill patients with thrombocytopenia and without high risk of spontaneous bleeding, we suggest transfusing platelets if platelet counts fall below 10 × 109/L (Conditional recommendation, very low certainty of evidence).
2. In stable non-bleeding critically ill patients with thrombocytopenia who are considered at high risk of spontaneous bleeding, we suggest transfusing platelets if platelet counts fall below 30-50 × 109/L (Conditional recommendation, very low certainty of evidence).
3. In critically ill patients with thrombocytopenia and serious active bleeding, we suggest transfusing platelets if platelet counts fall below 50 × 109/L (Conditional recommendation, very low certainty of evidence)
4. Vascular procedures
4 A. In critically ill patients at increased risk of bleeding due to thrombocytopenia undergoing a central venous catheter or arterial line insertion, we suggest against routine prophylactic platelet transfusion (Conditional recommendation, very low certainty of evidence).
4 B. In critically ill patients at increased risk of bleeding due to coagulopathy undergoing a central venous catheter or arterial line insertion, we suggest against routine prophylactic FFP transfusion (Conditional recommendation, very low certainty of evidence).
5. Bedside thoracic or abdominal procedure
5 A. In critically ill patients with increased risk of bleeding due to thrombocytopenia undergoing a bedside thoracentesis or paracentesis, we suggest against routine prophylactic platelet transfusion (Conditional recommendation, very low certainty of evidence).
5 B. In critically ill patients with increased risk of bleeding due to coagulopathy undergoing a bedside thoracentesis or paracentesis, we suggest against routine prophylactic FFP transfusion (Conditional recommendation, very low certainty of evidence).
6. Lumbar Puncture
6 A. In critically ill patients with increased risk of bleeding due to thrombocytopenia undergoing a bedside lumbar puncture, we suggest platelet transfusion if platelets counts are 40-50 × 109/L or lower (Conditional recommendation, very low certainty of evidence).
6 B. In critically ill patients with increased risk of bleeding due to coagulopathy undergoing a bedside lumbar puncture, we suggest FFP transfusion to target INR 1.5-2 before the procedure (Conditional recommendation, very low certainty of evidence).
7. Bedside Endoscopy
Bronchoscopy
7 A. In critically ill patients with increased risk of bleeding due to thrombocytopenia undergoing routine flexible bronchoscopy without biopsy, we suggest against routine prophylactic platelet transfusion (Conditional recommendation, very low certainty of evidence).
7 B. In critically ill patients with increased risk of bleeding due to coagulopathy undergoing a routine bedside flexible bronchoscopy without biopsy, we suggest against routine prophylactic FFP transfusion (Conditional recommendation, very low certainty of evidence).
GI Endoscopy
7 C. In critically ill patients with suspected portal hypertension related GI bleeding and increased risk of bleeding due to thrombocytopenia who are undergoing GI-endoscopy, we suggest against routine platelet transfusion (Conditional recommendation, very low certainty of evidence)
7 D. In critically ill patients with suspected portal hypertension related bleeding and increased risk of bleeding due to coagulopathy who are undergoing GI-endoscopy, we suggest against routine FFP transfusion (Conditional recommendation, very low certainty of evidence).
Reference:
Yataco, Angel Coz et al., Transfusion of Fresh Frozen Plasma and Platelets in Critically Ill Adults, CHEST Journal, DOI:10.1016/j.chest.2025.02.029
Powered by WPeMatico