Tiniest lung tumors that are hardest to reach can be diagnosed with robot-assisted bronchoscope
Powered by WPeMatico
Powered by WPeMatico
Powered by WPeMatico

Indianapolis:
In the Phase 3 EMBER-3 trial, Inluriyo reduced the risk of progression or death by 38% versus ET. Among patients with ESR1-mutated MBC, Inluriyo significantly improved progression-free survival (PFS) versus fulvestrant or exemestane, with a median PFS of 5.5 months vs 3.8 months (HR=0.62 [95% CI: 0.46-0.82]); p-value=0.0008.
Inluriyo is a treatment for ER+, HER2–, ESR1-mutated MBC. Some breast cancers develop ESR1 mutations that can cause estrogen receptors to become overactive and drive cancer growth. Inluriyo binds, blocks, and facilitates the degradation of these receptors, helping to slow disease progression. Its once-daily dosing provides patients with an oral treatment option.
“This therapy reflects our commitment to developing treatments that improve outcomes for people with breast cancer and represents an important step toward advancing innovative, all-oral treatment approaches,” said
The Inluriyo label contains a warning and precaution for embryo-fetal toxicity.
The FDA approval is based on the results of the EMBER-3 trial in the patient population harboring ESR1-mutated MBC (n=256). Patients received Inluriyo or ET as first-line treatment for MBC following recurrence on adjuvant aromatase inhibitor (AI), +/- prior CDK4/6 inhibitor (21%), or as second-line treatment for MBC following progression on AI, +/- prior CDK4/6 inhibitor (79%).
“This represents an important advancement for patients with ESR1-mutated MBC, a mutation found in nearly half of patients who have taken hormone therapies, often contributing to treatment resistance,” said
“The approval of Inluriyo expands the metastatic breast cancer treatment landscape for patients who test positive for the ESR1 mutation,” said
Inluriyo is also being studied in the ongoing Phase 3 EMBER-4 trial in the adjuvant setting for people with ER+, HER2– early breast cancer (EBC) at increased risk of recurrence, which is enrolling approximately 8,000 patients worldwide.
Inluriyo is expected to be available in
Powered by WPeMatico

Shujalpur: In a shocking case of bribery, a gynaecologist from Shujalpur has been accused of demanding and accepting a bribe from the family of a pregnant woman, despite conducting the delivery at the Shujalpur Government Hospital.
According to the complainant, his daughter had been under the doctor’s care throughout her pregnancy. He alleged that from the beginning, the doctor hinted that the delivery would require extra money and suggested shifting the procedure to her private clinic instead of the government hospital.
On September 18, the woman underwent a cesarean delivery at the government hospital. However, the complainant alleged that before proceeding with the surgery, the doctor demanded Rs 12,000. Unable to arrange the full amount immediately, he first paid Rs 8,000, but the doctor allegedly insisted that the remaining Rs 4,000 must be cleared quickly.
Also read- Retired Gynaecologist sentenced to 5 years in jail for bribery
After the surgery, the woman’s father claimed that the doctor refused to remove his daughter’s stitches until the pending money was paid. He said he was left humiliated and helpless, worrying not only about his daughter’s health but also about the safety of the newborn.
Speaking to Dainik Bhaskar, the father said, “I had already given Rs 8,000, but when I couldn’t arrange the balance, the doctor threatened not to cut my daughter’s stitches. What kind of system is this, where even in government hospitals you are forced to pay bribes?”
On September 23, the father claimed that he went to the doctor’s private clinic to make the payment. He said that he had to borrow the money from someone else. At the clinic, he first gave Rs 5,000 to the doctor’s assistant, and then another Rs 3,000 after about 20 minutes.
However, the woman’s father was clever; he recorded the money exchange scene with the assistant. The footage showed him handing over money while the assistant can be heard saying that the remaining amount would be settled during the stitch removal.
When asked about the allegations, the doctor denied all charges, calling them false and politically motivated. She said, “The accusations are false. I have never demanded any money. These claims are fabricated to tarnish my reputation.”
So far, no case has been registered by the police as the woman’s family has not yet filed any complaint against the doctor.
Also read- FIR against Haryana Health Director, 2 others for bribery and forgery allegations
Powered by WPeMatico

Hyderabad: Dr Reddy’s Labs has appointed Gayatri Prabhu as Head Digital Marketing (Emerging Markets).
Gayatri is a result-driven Digital Professional with 20 plus years of experience in driving Innovation, Digital Transformation and Business growth across Banking, Insurance and Pharma industry. She specialises in the areas of Transforming Offline Business to Digital, with key focus on New Customer Acquisition, Retention and Loyalty of Existing Customers, Customer Experience Management on Web, Mobile, Bots, Social Media, CRM, Marketing Automation and Analytics. She hold a master’s degree specializing in Marketing Management from NMIMS and a certification in ‘Leadership in Artificial Intelligence’ from ISB.
Read also: Dr Reddy’s Labs receives positive EMA Committee opinion for proposed biosimilar of Prolia, Xgeva
Established in 1984, Dr. Reddy’s Laboratories Ltd. is a global pharmaceutical company headquartered in Hyderabad, India. The Company offers a portfolio of products and services including APIs, generics, branded generics, biosimilars and OTC. Its major therapeutic areas of focus are gastrointestinal, cardiovascular, diabetology, oncology, pain management and dermatology. Dr Reddy’s Labs major markets include – USA, India, Russia & CIS countries, China, Brazil and Europe.
Read also: Dr Reddy’s Secures CDSCO Panel Approval to Import, Market Olutasidenib for AML
Powered by WPeMatico

Panaji: The All India Institute of Ayurveda in Goa on Wednesday launched a joint Integrative Oncology Research and Care Centre. The initiative combines modern oncology with AYUSH systems of medicine to improve the quality of life of cancer patients, an official said.
According to the PTI report, the inaugural clinic was conducted at the newly inaugurated Integrative Oncology Research and Care Centre of AIIA in Dhargal of North Goa, the senior official said.
He said four complex cancer cases were presented before a multi-disciplinary panel comprising oncologists, AYUSH (Ayurveda, Yoga and Naturopathy, Unani, Siddha, and Homoeopathy) experts, and senior researchers.
Also Read:Ministry of Ayush celebrates 10th Ayurveda Day
Patients and their relatives were present during the deliberations and had their queries addressed in detail.
“Each case was evaluated from diverse clinical perspectives, and after comprehensive discussion, the panel reached a consensus on therapeutic and adjuvant treatment options. The initiative aims to offer patients an evidence-based integrative approach to improve treatment outcomes and quality of life,” the official said.
“This is the first-of-its-kind collaboration in India, and it is significant that Goa has taken the lead,” said Dr Shekhar Salkar, senior oncosurgeon at Manipal Hospital, Goa, and member of an empowered committee.
He added that such clinics will now be held every month, with prior appointments required at the AIIA centre.
The clinic brought together experts from various institutions, including Tata Memorial Centre and its Navi Mumbai-based R&D wing ACTREC.
According to organisers, the effort represents a shared vision of modern and traditional medicine experts to provide holistic cancer care. Patients attending the first session reported being “immensely benefited” from the exercise, officials said.
Medical Dialogues had earlier reported that Patients visiting the South Goa District Hospital will no longer have to undergo treatment on wheelchairs or stretchers, as the hospital has finally been upgraded to its full 500-bed capacity.
Also Read:AIIA holds bike rally under the theme Ayurveda for People and Planet
Powered by WPeMatico
Hungary: Researchers have found in a new study that once-weekly semaglutide significantly reduces weight and HbA1c while favorably modifying atherogenic lipoprotein subfractions in type 2 diabetes patients. After 52 weeks, semaglutide lowered LDL and non-HDL cholesterol, reduced small dense LDL, and increased large, cardioprotective HDL particles. In contrast, sitagliptin showed only modest glycemic and weight-lowering benefits with minimal impact on lipid or inflammatory markers.
The findings, published in the International Journal of Molecular Sciences, are the result of a 52-week randomized clinical trial led by László Imre Tóth and colleagues from the Division of Metabolism, Department of Internal Medicine, University of Debrecen, Hungary. The study compared the effects of semaglutide, a glucagon-like peptide-1 receptor agonist (GLP-1 RA), with sitagliptin, a dipeptidyl peptidase-4 (DPP-4) inhibitor, in individuals with type 2 diabetes mellitus (T2DM).
A total of 34 obese adults with T2DM were enrolled and randomized to receive either once-weekly subcutaneous semaglutide (n=18) or once-daily oral sitagliptin (n=16). An additional 31 age- and weight-matched non-diabetic individuals were included as controls. The study primarily assessed changes in glycemic control, anthropometric measurements, and detailed lipoprotein subfractions using Lipoprint gel electrophoresis.
The study revealed the following findings:
The authors emphasize that semaglutide may play a critical role in managing not just glycemic control, but also broader cardiometabolic risks associated with type 2 diabetes. By promoting a healthier lipid profile, it may contribute to lowering cardiovascular disease risk in this population.
“These findings highlight the therapeutic potential of semaglutide in addressing multiple facets of T2DM and support further research into its long-term cardiovascular benefits across diverse patient populations,” the authors concluded.
Reference:
Tóth, L. I., Harsányi, A., Csiha, S., Molnár, Á., Lőrincz, H., Nagy, A. C., Paragh, G., Harangi, M., & Sztanek, F. (2024). Semaglutide Improves Lipid Subfraction Profiles in Type 2 Diabetes: Insights from a One-Year Follow-Up Study. International Journal of Molecular Sciences, 26(13), 5951. https://doi.org/10.3390/ijms26135951
Powered by WPeMatico

Researchers have identified in a new study that the C-reactive protein–triglyceride glucose index (CTI) is linearly and positively correlated with stroke risk among subjects with cardiovascular-kidney-metabolic (CKM) syndrome. The study concluded that CTI not only better predicts stroke risk compared to the well-established triglyceride–glucose index (TyG), but also represents a useful risk marker among patients with CKM stage 2 and 3, where the risk of stroke events is notably high. The study was published in BMC Cardiovascular Disorders by Yinsong Xu and fellow researchers.
The data from 5767 subjects with stages 0–3 CKM syndrome participating in the China Health and Retirement Longitudinal Study (CHARLS) were examined in this study. The participants were tracked from 2011 to 2020 to examine the development of incident stroke, as diagnosed by self-reported questionnaires.
The CTI was derived from the formula:
CTI = 0.412 × ln[hs-CRP (mg/L)] + ln[(triglyceride (mg/dL) × fasting glucose (mg/dL)) / 2]
More advanced statistical techniques such as Cox proportional hazards models, restricted cubic spline (RCS) analysis, and subgroup analyses were utilized to compare associations of CTI with risk of stroke. Predictive accuracy was compared with TyG and metabolic score for insulin resistance (METS-IR) using time-dependent ROC analysis. Multiple testing correction was achieved through the false discovery rate approach to provide robustness.
Results
After controlling for possible confounders, higher levels of CTI were significantly linked to increased risk of stroke (Hazard Ratio [HR] 1.33, 95% CI 1.18–1.51).
RCS analysis revealed a linear relationship, without any suggestion of nonlinearity (P for nonlinearity = 0.182).
Subgroup analysis showed stage-specific effects:
CKM Stage 2: HR 1.27, 95% CI 1.04–1.56 (P = 0.019; adjusted P = 0.023)
CKM Stage 3: HR 1.25, 95% CI 1.04–1.50 (P = 0.022; adjusted P = 0.025)
CKM Stages 0–1: No significant association seen
Predictive performance analysis also revealed that CTI outperformed TyG at both year 5 and year 7 in stroke prediction consistently, with DeLong’s test adjusted P = 0.028 for both contrasts.
The present nationwide cohort study supplies strong evidence that the CTI is independently and linearly related to stroke risk in CKM syndrome patients, especially at stages 2 and 3. In addition, CTI always presents higher predictive accuracy than TyG and thus has the potential to serve as an excellent clinical tool for risk stratification and early intervention in high-risk individuals.
Reference:
Xu Y, Chen S, Zhu J, et al. C-reactive protein-triglyceride glucose index and stroke risk in early cardiovascular-kidney-metabolic syndrome: a National cohort study. BMC Cardiovasc Disord. 2025;25(1):634. Published 2025 Aug 26. doi:10.1186/s12872-025-05143-3
Powered by WPeMatico
Researchers have found in a new clinical trial that a single dose of clesrovimab, a long-acting investigational monoclonal antibody targeting site IV of the respiratory syncytial virus (RSV) fusion protein, significantly reduced the risk of RSV-associated illness in healthy infants. The study, published in The New England Journal of Medicine, evaluated the safety and efficacy of clesrovimab in both preterm and full-term infants during their first RSV season, a period marked by heightened vulnerability to severe lower respiratory tract infections. Infants were randomly assigned to receive either one intramuscular injection of clesrovimab or placebo, and outcomes were monitored for 150 days following administration. Findings showed that clesrovimab markedly lowered the incidence of medically attended lower respiratory tract infections due to RSV compared with placebo, and also substantially reduced RSV-related hospitalizations, underscoring its potential as a preventive strategy for this common and sometimes life-threatening infection. Importantly, the safety profile of clesrovimab was comparable to placebo, with no signal of increased adverse events, further supporting its potential suitability for widespread use. RSV is a leading cause of infant hospitalization worldwide, and while supportive care remains the mainstay of treatment, there is a recognized need for effective preventive measures beyond maternal vaccination and existing monoclonal antibodies. The results of this large, randomized, placebo-controlled trial suggest that clesrovimab could fill an important gap in infant RSV prevention strategies by providing durable protection with a single dose, potentially easing the burden on healthcare systems and reducing parental anxiety during RSV season. The authors noted that longer follow-up and real-world studies will be essential to confirm durability of protection, cost-effectiveness, and feasibility of integration into national immunization programs, but the current evidence positions clesrovimab as a promising candidate for future inclusion in RSV prevention protocols for infants.
Keywords: Clesrovimab, RSV, monoclonal antibody, infants, prevention, respiratory infection, hospitalization, immunization
Reference: Zar HJ, Simões EAF, Madhi SA, Ramilo O, Senders SD, Shepard JS, Laoprasopwattana K, et al; CLEVER (MK-1654-004) Study Group. Clesrovimab for Prevention of RSV Disease in Healthy Infants. N Engl J Med. 2025; DOI: 10.1056/NEJMoa2502984.
Powered by WPeMatico
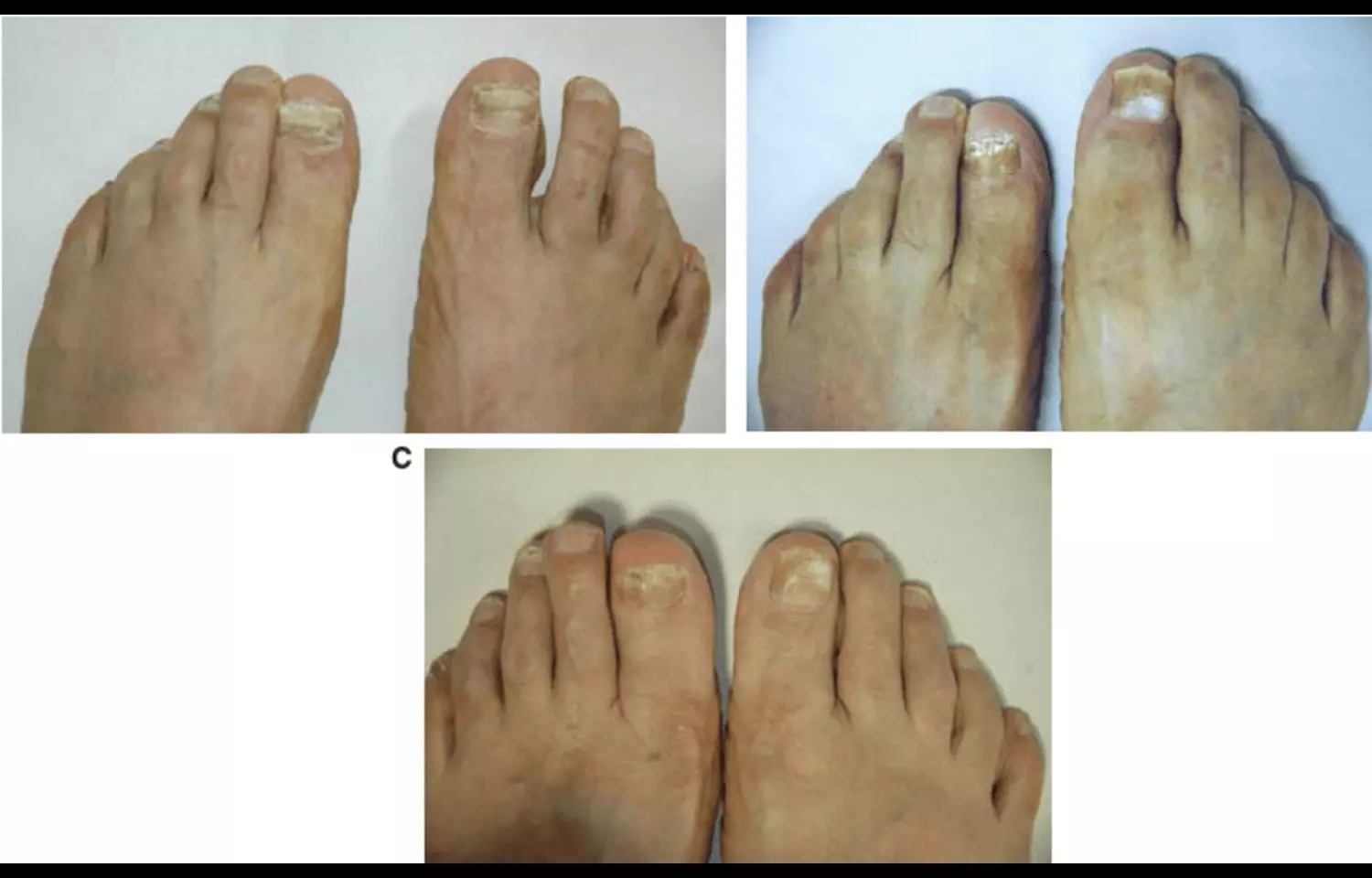
Researchers have found in a new study that adding two percent crisaborole ointment to amorolfine hydrochloride cream significantly enhances therapeutic outcomes in the management of keratinized tinea foot. Tinea foot, a common superficial fungal infection caused by dermatophytes, often presents in a chronic keratinized form where thickened skin and scaling limit the penetration and efficacy of topical antifungal treatments. Amorolfine hydrochloride, a well-established antifungal agent, is effective against dermatophytes but faces challenges in achieving optimal results in heavily keratinized lesions due to poor drug penetration. The study explored whether the phosphodiesterase-4 (PDE4) inhibitor crisaborole, known for its anti-inflammatory and skin barrier–modulating properties, could improve outcomes when combined with amorolfine. The combination therapy demonstrated superior antifungal activity, greater improvement in lesion clearance, and better patient-reported symptom relief compared with amorolfine monotherapy. The enhanced effectiveness is thought to stem from crisaborole’s ability to reduce inflammation, soften hyperkeratotic tissue, and improve drug absorption, thereby allowing amorolfine to penetrate more effectively into the infected areas. Importantly, the combination was well tolerated, with no significant increase in adverse effects, supporting its safety for clinical use. These findings suggest that dual therapy with crisaborole and amorolfine may provide a more effective option for patients with keratinized tinea foot, a condition that is often resistant to standard topical treatments and requires prolonged therapy. The results also open avenues for exploring similar combination approaches in other keratinized fungal infections where topical antifungal penetration is limited. Clinicians may consider the combined regimen as part of personalized treatment strategies, particularly for patients with chronic or recurrent infections. However, the researchers emphasized the need for larger randomized controlled trials to validate these results, assess long-term efficacy, and determine whether the combination can reduce recurrence rates.
Keywords: Crisaborole, amorolfine, keratinized tinea foot, dermatophyte infection, topical antifungal therapy, combination treatment, PDE4 inhibitor, skin barrier, hyperkeratosis, clinical effectiveness
Powered by WPeMatico
