Coaches can boost athletes’ mental health by being ‘authentic leaders’
Powered by WPeMatico
Powered by WPeMatico
Powered by WPeMatico

Journal of Neonatal Critical Care and Anesthesia is an open-access peer-reviewed journal committed to publishing high-quality articles in the field of Neonatal Anesthesia and common health problems of newborns and neonates. The journal is owned by the NAS (Neonatal Anesthesia Society) and published by the Scientific Scholar.
Powered by WPeMatico

Ahmedabad: Torrent Pharma has announced that the US Food and Drug Administration (USFDA) has issued
an Establishment Inspection Report (“EIR”) for the Company’s Oral-Oncology manufacturing facility situated in Bileshwarpura, Gujarat. The inspection
has now been successfully closed by the USFDA.
Torrent Pharma is an Indian multinational pharmaceutical company headquartered in Ahmedabad, India. The company specializes in therapeutic segment of cardiovascular (CV), central nervous system (CNS), gastro-intestinal (GI) and women healthcare (WHC).
Read also: Torrent Pharma reports 52 percent increase in profit after tax to Rs 443 crore for Q3
Powered by WPeMatico

New Delhi: The viral social media post claiming that Nashik-based Pulmonologist Dr. Kailash Rathi was attacked by a “Jihadi”, a Muslim person using a sickle is a fake.
While the viral post on X (formerly Twitter) is trying to give the issue a communal angle, the reality is that Dr. Rathi was brutally attacked inside a hospital in Maharashtra over a monetary dispute by the husband of a former colleague, a Hindu man.
The horror was captured on a CCTV camera and the viral video shows that the attacker struck the 49-year-old doctor around 18 times with a sickle on last week. Thereafter, the doctor, a chest physician and critical care specialist, was admitted to a local hospital in a critical condition.
The Claim:
The disturbing video of the accused attacking Dr. Rathi went viral on social media as members of the medical fraternity and general public alike expressed their shock over the brutality of the attack.
However, one such viral post claimed that the doctor was attacked by in Nashik’s Suyog Hospital by a Jihadi attacker, giving it a communal look. The rough Hindi-to-English translation of the X post states, “Take heart and watch today’s most heart-wrenching video••••••••* *In Maharashtra, Nashik’s Suyog Hospital, a Jihadi attacker entered the ICU and attacked Dr. Kailash Rathi with a sharp weapon, due to multiple indiscriminate attacks in 30 seconds.”
Fact Check:
Medical Dialogues had earlier reported about the brutal and horrifying attack on Dr. Rathi. The Panchavati police arrested the accused, who has been identified as Rajendra More, a Hindu. The police secured custody for seven days after producing him in the city court.
The accused and his wife, who is a former colleague of the doctor, have been booked by police based on a complaint of the victim’s wife.
Therefore, the claim that the doctor was attacked by a “Jihadi”, a Muslim man is misleading. There was no communal reason for the attack. In reality, the doctor was attacked by the husband of a former colleague, who is Hindu by religion.
Powered by WPeMatico

Wayanad: In an unexpected move, the Directorate of Medical Education has instructed the transfer of a group of seven senior resident doctors from the Government Medical College Hospital (GMCH) Kozhikode and Government General Hospital to the Government Medical College Hospital (GMCH) Wayanad for a month, starting from February 22 to May 21.
This sudden decision has reportedly intensified the strain on patient care at the two healthcare institutions in Kozhikode, which were already grappling with a shortage of doctors.
Considering that certain departments are left with only two doctors now, the hospital management has come up with a strategic move of assigning days to particular works to handle the work pressure. On Monday, Wednesday, and Friday outpatient (OP) services will be available while surgeries will take place only on Tuesday, Thursday and Saturday.
Previously patients could avail OP services 6 days a week and the hospital could accommodate 150 to 180 surgeries in a month. The ENT department of the hospital is also grappling with the shortest of doctors after the retirement of a senior doctor. The creation of new posts and appointment of more doctors are being demanded by the doctors considering the increasing number of patients in the facility.
Also Read: Private Practice: GMC Kozhikode Nephrologist faces suspension
Powered by WPeMatico
Researchers at the National Institutes of Health have identified antibodies targeting a hard-to-spot region of the influenza virus, shedding light on the relatively unexplored “dark side” of the neuraminidase (NA) protein head. The antibodies target a region of the NA protein that is common among many influenza viruses, including H3N2 subtype viruses, and could be a new target for countermeasures. The research, led by scientists at the National Institute of Allergy and Infectious Diseases’ Vaccine Research Center, part of NIH, was published today in Immunity..
Influenza, or flu, sickens millions of people across the globe each year and can lead to severe illness and death. While vaccination against influenza reduces the burden of the disease, updated vaccines are needed each season to provide protection against the many strains and subtypes of the rapidly evolving virus. Vaccines that provide protection against a broad range of influenza viruses could prevent outbreaks of new and reemerging flu viruses without the need for yearly vaccine reformulation or vaccinations.
One way to improve influenza vaccines and other countermeasures is to identify new targets on the virus’s surface proteins in “conserved” regions-portions that tend to be relatively unchanged between different strains of the virus. Influenza NA is a surface protein containing a globular head portion and a narrow stalk portion. The underside of the NA head contains a highly conserved region with targets for antibodies-known as epitopes-that make it vulnerable to antibody binding and inhibition of the virus, as well as not being impacted by mutations common in drug-resistant strains. This region is termed the “dark side” due to its partially hidden location and relatively unexplored characteristics.
The researchers isolated human antibodies that target the NA dark side from the blood of two people who had recovered from influenza type A subtype H3N2, a major subtype of seasonal flu viruses. In lab tests, the antibodies inhibited propagation of viruses from subtype H2N2, the subtype that caused pandemic influenza in 1957-58, and H3N2 viruses from humans, swine, and birds. The antibodies also protected mice from lethal infection by a subtype H3N2 virus when given to the animals either one day before or two days after infection, showing that the antibody may treat and prevent influenza in this model.
The scientists analyzed the structure of two of the antibodies while bound to NA using advanced microscopy techniques known as cryogenic electron microscopy. Each antibody targeted different, nonoverlapping regions of the dark side, demonstrating that this region has multiple areas that may be useful to explore for countermeasure development.
These findings show that the NA dark side has unique, previously untapped epitopes that could be applied to the development of new vaccine and therapeutic strategies. They suggest that antibodies targeting the NA dark side could be useful in combination with antivirals or other types of antibodies for interventions against influenza, as they are effective against influenza viruses with drug-resistant mutations. The researchers also note that NA dark side targets could be included in the next generation of broadly protective vaccines against influenza.
Reference:
Lederhofer, J. et al. Protective human monoclonal antibodies target conserved sites of vulnerability on the underside of influenza virus neuraminidase. Immunity. DOI: 10.1016/j.immuni.2024.02.003(link is external) (2024).
Powered by WPeMatico

New Delhi: Considering the requirement of quality in-patient care, Ayush hospitals should be spread across the country following the bed per population norm, according to a set of new guidelines issued by the Ayush Ministry.
According to the norm, one hospital bed per 5,000 population is “essential” while 1 bed per 2,000 population is “desirable”.
There are 3,844 Ayush hospitals in various categories across the country run by central and state governments. These hospitals together have a bed strength of 60,943.
The “essential” number of beds in Ayush hospitals may be provided through the public health system such as medical colleges, district hospitals, 10-bedded, 30-bedded, 50 or more bedded Ayush hospitals, the Indian Public Health Standards (IPHS) for Ayush healthcare facilities stated.
To achieve the “desirable” number of beds, the contribution of the private sector may also be considered while continuing to strengthen and increase bed provision at public health facilities, the guidelines said.
Also Read:Strenghthen AYUSH programmes to enhance quality of lives of people: Shri Sarbanand Sonowal
As a thumb rule, all beds available and functional for a patient for more than 24 hours have been calculated as in-patient hospital beds.
The Ayush hospitals, in adherence to IPHS standards, are expected to provide a comprehensive range of essential services that align with the principles of Ayurveda, Yoga, Unani, Siddha, SowaRigpa and Homoeopathy, the document said.
This includes but is not limited to promotive, preventive, curative, and rehabilitative services catering to a wide spectrum of health conditions.
These facilities are encouraged to integrate traditional and complementary medicine practices with the contemporary healthcare system, fostering a harmonious blend that caters to the diverse healthcare needs of the population, the document said.
The guidelines also stressed ensuring a continuum of care, establishment of assured referral systems along with facility readiness to manage referred cases.
All Ayush healthcare facilities should be strategically situated to ensure convenient access for the rural and urban communities they serve, it stated, adding that adequate space and infrastructure should be allocated to cater to the evolving healthcare needs.
All Ayush hospitals should be resilient to climatic and environmental changes and able to handle sudden healthcare needs during disasters and emergencies, epidemics and pandemics.
The IPHS guidelines for Ayush hospitals has emphasised strategic planning for service delivery as a priority, before investing in other components such as infrastructure, human resources, drugs, diagnostics, equipment, and others.
To achieve this, the set of guidelines has categorised healthcare services as either ‘essential’ or ‘desirable’ and correspondingly classified human resources in a similar fashion.
According to the guidelines, states and Union territories should conduct Ayush-specific training needs assessment to identify the training requirements of the Ayush healthcare workforce.
Apart from this, identify the need for integrating Ayush and modern medicine training for Ayush healthcare professionals, if required, the document said, adding that a robust grievance redressal mechanism should be established in all such hospitals.
Every state and Union Territory should have an occupational safety and health policy for Ayush hospitals, outlining the framework for ensuring the safety, health and well-being of employees, patients, and visitors.
The document stated that quality plays an important role and it should ideally be maintained in such a manner that the outcome meets the prescribed standards, even without any supervision.
Any proposed system must incorporate best practices from contemporary systems like the National Quality Assurance System.
National Accreditation Board for Hospital and Healthcare Providers standards for various Ayush systems will be referred and consulted to customise them for meeting the needs of Ayush Centers, the document said.
Powered by WPeMatico

USA: The researchers from Rice University in Texas, US, have developed a rapid, accurate test that could be a game changer for diagnosing malaria, especially in areas with limited access to healthcare facilities.
The test, which is significantly faster and easier to use than traditional tests, can help healthcare providers quickly identify and treat severe cases, potentially saving lives. By enabling early detection and appropriate management of malaria cases, the disease burden can be reduced and can improve patient outcomes in Africa and beyond.
The work is published in the journal Biosensors and Bioelectronics.
Malaria remains a significant global health challenge with an estimated 247 million cases and more than 600,000 deaths annually, the majority of which occur in sub-Saharan Africa. The most severe form, cerebral malaria, has a high mortality rate, particularly in children under 5 years of age.
Current rapid diagnostic tests (RDTs) for malaria provide only a binary result-positive or negative-often missing asymptomatic infections and lacking the sensitivity needed to detect severe cases early. Molecular assays, while more sensitive, are expensive, time-consuming and require specialized equipment and trained personnel, making them impractical for widespread use in resource-limited settings.
To address these challenges, a team led by mechanical engineer Peter Lillehoj of Rice’s Brown School of Engineering, created a microfluidic point-of-care (mPOC) immunoassay for quantifying a malaria parasite biomarker, Plasmodium falciparum histidine-rich protein 2 (PfHRP2), in whole blood.
The device features two diagnostic modes for detecting PfHRP2 at low and high concentrations, making it useful for various diagnostic applications, including the detection of asymptomatic infection and the prediction of disease outcomes. Test results are available in just 15 minutes and can be accessed on a smartphone app, developed by the research team.
“The mPOC immunoassay was designed to be simple, accurate and field-deployable, making it suitable for use in rural and remote health centers in sub-Saharan Africa,” said Lillehoj, associate professor of mechanical engineering. “Unlike traditional tests, this device does not require plasma separation, pipetting, complicated sample processing or long incubations, making it easy to use even by minimally trained health care providers.”
In testing conducted in Malawi, the mPOC immunoassay demonstrated similar accuracy to a commercial PfHRP2 enzyme-linked immunosorbent assay (ELISA) test, while being 12 times faster and simpler to use. The ability to quickly and accurately diagnose malaria, particularly cerebral malaria, at the point of care could lead to early identification and treatment of severe cases, according to Lillehoj.
“In areas with limited access to health care facilities, our test could be a game-changer,” he said. “It can help health care providers quickly identify and treat severe cases, potentially saving lives. By enabling early detection and appropriate management of malaria cases, we can reduce the burden of the disease and improve patient outcomes in Africa and beyond.”
Reference:
Li J, Saidi AM, Seydel K, Lillehoj PB. Rapid diagnosis and prognosis of malaria infection using a microfluidic point-of-care immunoassay. Biosens Bioelectron. 2024 Apr 15;250:116091. doi: 10.1016/j.bios.2024.116091.
Powered by WPeMatico
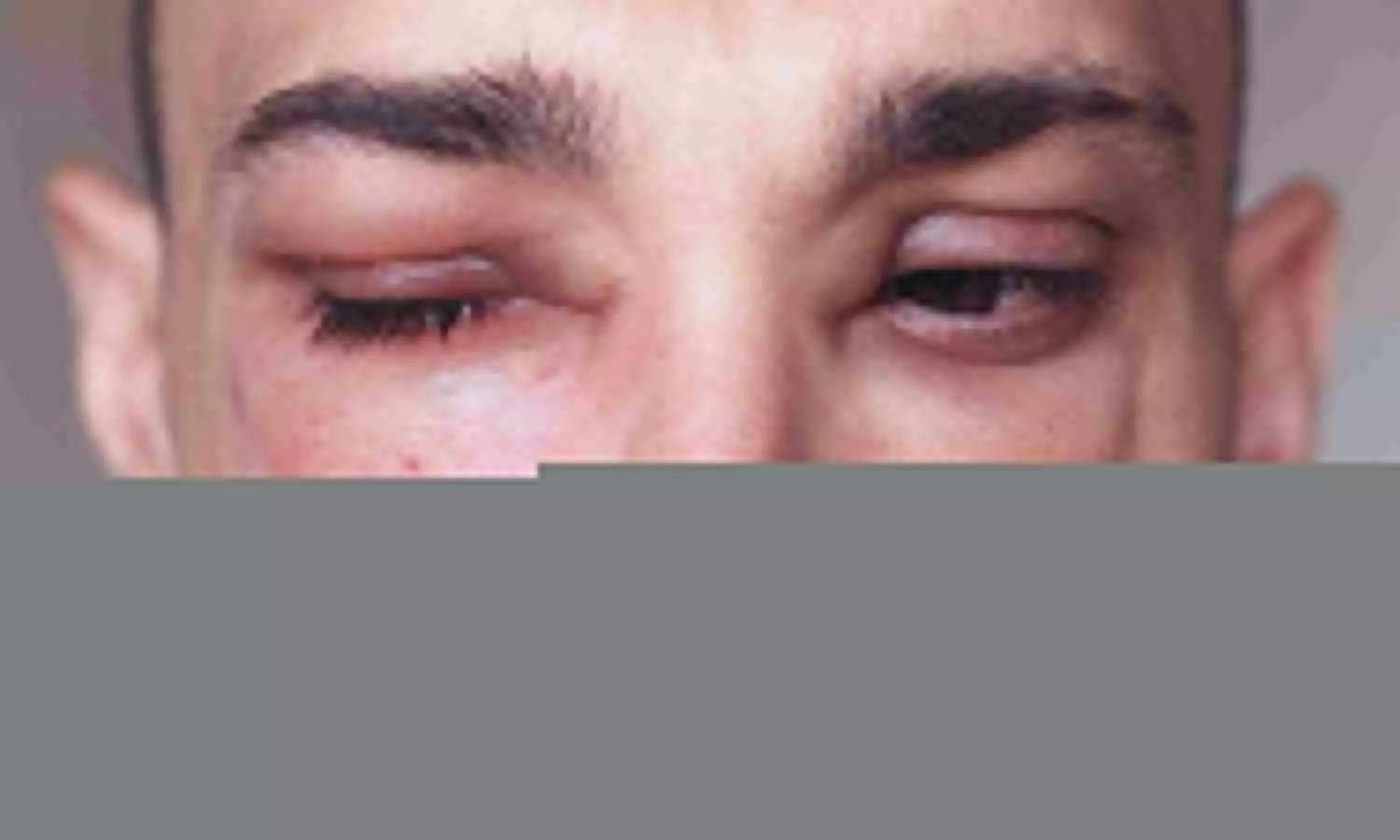
USA: A previous phase 2 open-label extension (OLE) study of donidalorsen in patients with Type 1/2 hereditary angioedema (HAE) showed a 76% reduction in monthly attack rate and improved quality-of-life (QoL) with every eight weeks (Q8W) dosing at Year 1.
A recent article published in The Journal of Allergy and Clinical Immunology reports the Year 2 results of patients who received donidalorsen Q8W.
Michael Manning, Allergy, Asthma & Immunology Associates, and colleagues reported that at Year 2, donidalorsen Q8W was well-tolerated, had plasma prekallikrein levels similar to Q4W dosing, and showed durable efficacy in hereditary angioedema attack reduction. The findings supported the continued study of Q8W dosing.
Hereditary angioedema is an inherited condition with characteristics of recurrent episodes of nonpitting, nonpruritic, subcutaneous or submucosal swelling without urticarial lesions. Multiple body areas, including feet, hands, genitalia, intestinal wall, face, larynx, or tongue, can be involved.
There exist two main types of HAE that are inherited in an autosomal dominant manner. Type I HAE accounts for about 85% of cases and results from a quantitative deficiency of C1 inhibitor. Type II HAE is responsible for about 15% of cases and results from a dysfunctional C1 inhibitor protein. Donidalorsen is an investigational ligand-conjugated antisense oligonucleotide designed for hepatic uptake and inhibition of prekallikrein production.
Dr. Manning and the team presented the two-year update on the impact of donidalorsen taken every 8 weeks in patients with HAE.
The OLE study had fixed (Weeks 1–16, donidalorsen 80 mg subcutaneously every 4 weeks [Q4W]) and flexible (Weeks 17–105, donidalorsen 80 mg Q4W, 80 mg Q8W, or 100 mg Q4W) dosing periods.
Based on the analysis, the researchers reported the following findings:
“At Year 2, donidalorsen Q8W was well-tolerated, had plasma prekallikrein levels comparable to Q4W dosing, and showed durable efficacy in HAE attack reduction, supporting the continued study of Q8W dosing,” the researchers wrote.
Reference:
DOI: https://doi.org/10.1016/j.jaci.2023.11.031
Powered by WPeMatico
