Dapagliflozin reduced the risk of all-cause mortality or worsening heart failure by 28% in patients with aortic stenosis undergoing transcatheter aortic valve implantation (TAVI), a new study has reported.
The DapaTAVI trial, a multicenter randomized controlled study published in The New England Journal of Medicine, assessed dapagliflozin 10 mg once daily in 1222 high-risk patients undergoing TAVI across 39 centers in Spain. Participants had severe aortic stenosis, prior heart failure, and at least one additional risk factor—moderate renal impairment (eGFR 25–75 ml/min/1.73 m²), type 2 diabetes, or LVEF ≤40%; the mean age was 82.4 years, with 72% aged ≥80 years. The primary endpoint was a composite of all-cause mortality or worsening heart failure at one year. The trial’s primary endpoint was a composite of all-cause mortality or worsening heart failure (hospitalization or urgent visits requiring IV diuretics) at one-year follow-up.
Key findings of the study include
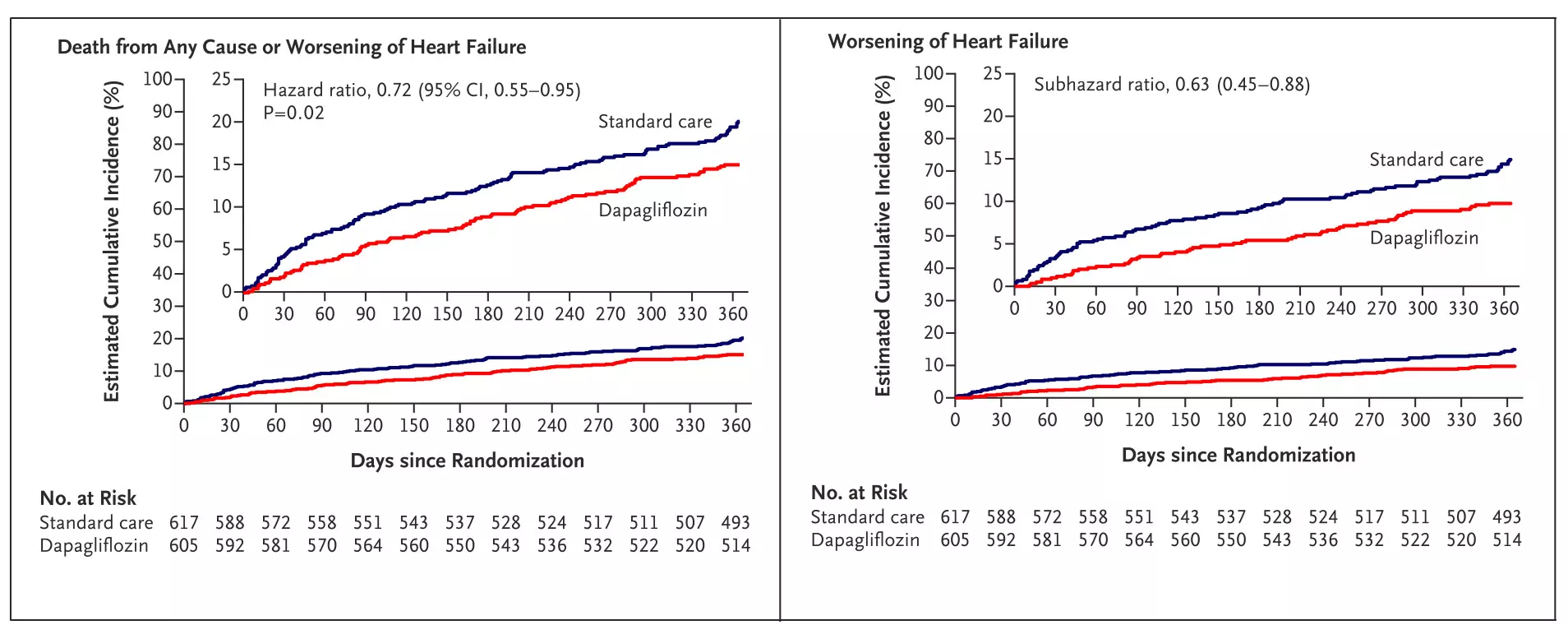
At one year, dapagliflozin reduced the primary composite outcome of all-cause mortality or worsening heart failure by 28% relative risk reduction (15.0% dapagliflozin vs 20.1% standard care; HR 0.72; 95% CI, 0.55–0.95; P = 0.02).
Figure: Kaplan–Meier curve showing time to primary composite outcome (death or worsening of heart failure) post-TAVI.Adapted from Raposeiras-Roubín S, et al. Dapagliflozin in Patients Undergoing Transcatheter Aortic-Valve Implantation. N Engl J Med. 2025; DOI: 10.1056/NEJMoa2500366.
Worsening heart failure was reduced by 37% (9.4% vs 14.4%; subHR 0.63; 95% CI, 0.45–0.88), hospitalizations for heart failure by 32% (7.4% vs 10.7%; subHR 0.68; 95% CI, 0.46–0.99), and urgent heart failure visits requiring IV diuretics by 54% (2.8% vs 6.0%; subHR 0.46; 95% CI, 0.26–0.82).
Dapagliflozin also reduced the secondary outcome of cardiovascular death or heart failure hospitalization by 29% (10.1% vs 13.8%; subHR 0.71; 95% CI, 0.51–0.98). The total number of recurrent events—cardiovascular deaths and heart failure hospitalizations—was reduced by 33% (79 vs 121 events; rate ratio 0.67; 95% CI, 0.47–0.95).
A prespecified subgroup analysis was performed to assess the consistency of treatment effect, with key results summarized below.
Benefit in Patients with CV Risk Factors: In patients with type 2 diabetes, dapagliflozin reduced the primary outcome by 33% (15.2% vs 22.7%; HR, 0.63; 95% CI, 0.42–0.94). In those with hypertension, the primary endpoint outcome reduction was 24% (15.3% vs 20.2%; HR 0.73; 95% CI, 0.54–0.97).
Outcome in Patients with Cardiac Co-Morbidities: In patients with preserved left ventricular ejection fraction (LVEF >40%), dapagliflozin reduced primary outcome by 33% (14.1% vs 21.2%; HR 0.67; 95% CI, 0.50–0.91). Those with mild or no left ventricular hypertrophy also benefited, with a 29% event reduction (11.8% vs 16.1%; HR 0.72; 95% CI, 0.52–1.00).
Patients with atrial fibrillation had a 33% relative risk reduction (19.6% vs 29.2%; HR 0.63; 95% CI, 0.44–0.90).
Renal Function: In patients with impaired renal function (eGFR <60 ml/min/1.73 m²), dapagliflozin reduced primary outcome by 28% (17.3% vs 22.9%; HR 0.71; 95% CI, 0.49–1.03). Among those with preserved kidney function (eGFR ≥60), the risk was reduced by 26% (12.7% vs 17.3%; HR 0.72; 95% CI, 0.45–1.16).
Background Cardiovascular Therapy: Dapagliflozin reduced the composite outcome of death or worsening heart failure by 30% in patients receiving RAS inhibitors (12.4% vs 17.6%; HR 0.68; 95% CI, 0.47–0.99). While not statistically significant, similar trends were observed in patients on beta-blockers (19.2% vs 24.9%; HR 0.74; 95% CI, 0.50–1.10) and diuretics (16.8% vs 21.6%; HR 0.75; 95% CI, 0.56–1.02), indicating a consistent treatment effect across therapies.
The results of DapaTAVI trial highlight a promising role for the SGLT2 inhibitor, dapagliflozin, in patients undergoing TAVI, particularly those with coexisting heart failure or renal dysfunction.
Reference: Díez-Villanueva, Pablo, et al. “Dapagliflozin in Patients Undergoing Transcatheter Aortic Valve Implantation.” The New England Journal of Medicine, vol. 390, no. 13, 2025, pp. 1234–1245. DOI: 10.1056/NEJMoa2500366.
Abbreviations: TAVI – Transcatheter Aortic Valve Implantation, DapaTAVI – Dapagliflozin in Patients Undergoing Transcatheter Aortic Valve Implantation, HR – Hazard Ratio, subHR – Subdistribution Hazard Ratio, CI – Confidence Interval, eGFR – Estimated Glomerular Filtration Rate, LVEF – Left Ventricular Ejection Fraction, IV – Intravenous, SGLT2 – Sodium-Glucose Cotransporter 2, RAS – Renin-Angiotensin System