Survey of US parents highlights need for more awareness about newborn screening, cystic fibrosis
Powered by WPeMatico
Powered by WPeMatico
Powered by WPeMatico

Vadodara: Alembic Pharma has announced that the Company has received an
Establishment Inspection Report (EIR) from the US Food and Drug Administration (US
FDA) for the inspection carried out at the Oncology (Injectable and Oral Solid)
Formulation Facility (F-2) at Panelav.
The USFDA had inspected the facility from 28th February, 2024 to 8th March, 2024 and issued a Form 483 with four procedural observations.
“With this, for all our USFDA facilities, EIRs are in place,” the Company stated in a BSE filing.
Read also: Alembic Pharma gets tentative USFDA nod for breast cancer drug Ribociclib in March Quarter
Headquartered in Vadodara, Gujarat, Alembic Pharmaceuticals Limited is involved in manufacturing and marketing India Formulations, International Generics, and Active Pharmaceutical Ingredients with vertical integration capabilities. The company was founded in 1907. Alembic’s state of the art research and manufacturing facilities are approved by regulatory authorities of many developed countries including the USFDA.
Read also: Alembic Pharma Slashes the Price of Tofastar by 50% for Benefit of Rheumatoid Arthritis Patients
Powered by WPeMatico

New Delhi: Glenmark and Cipla are recalling products from the American market over manufacturing issues, as per the US health regulator.
According to the latest Enforcement Report released by the US Food and Drug Administration (USFDA), Cipla’s New Jersey-based subsidiary is recalling 59,244 packs of Ipratropium Bromide and Albuterol Sulfate Inhalation Solution.
Powered by WPeMatico

Patna: The Patna High Court dismissed a petition seeking an increase in the
maximum age limit for appointing assistant professors in government medical
colleges in Bihar. On Monday, the decision was reached by a division
bench comprising Chief Justice K Vinod Chandran and Justice Harish Kumar while
dismissing the writ petition filed by Dr Nishant.
The petitioner had contested the legality of Rule 7 of the Senior
Resident/Tutor & Bihar Medical Education Service (Recruitment, Appointment,
and Promotion) Rules, 2008, which sets the maximum age limit for unreserved
candidates at 45 years, for other backward castes at 48 years, and for
scheduled castes and tribes at 50 years, for the appointment to assistant
professor positions in government medical colleges in Bihar, reports the Times of India.
Dr Nishant’s counsel, Kumar Kaushik, argued that the state’s age limit
should match that set by the National Medical Commission (NMC), India’s
regulatory body for the medical profession and education. The NMC has
established the maximum age limit for appointing assistant professors in
medical colleges at 70 years. However, the court upheld the existing
rules and dismissed the petition.
The age limit has been a persistent problem for many doctors. In a move that will bring relief to thousands of doctors wanting to start their careers in the field of medical education, the National Medical Commission (NMC) might soon do away with the age limit of 45 years prescribed for the initial appointment of the Senior Residents. The Postgraduate Medical Education Board (PGMEB) of NMC examined the cases of the non-medical postgraduate students with MSC (Medical) degrees and decided to recommend to NMC abolishing the age limit of 45 years prescribed for the Senior Residents in the Teachers Eligibility Qualifications in Medical Institutions Regulations, 2022.
Powered by WPeMatico
USA: In a groundbreaking development for cardiovascular medicine, the results of the ALIGN-AR study have sparked hope for patients suffering from high-risk symptomatic native aortic regurgitation. The prospective, multicentre, single-arm study focused on Transcatheter Aortic Valve Implantation (TAVI) as a potential solution for this challenging condition.
The study showed that using a dedicated transcatheter heart valve for patients with symptomatic moderate-to-severe or severe aortic regurgitation (AR) at high risk of complications or mortality after surgical aortic valve replacement (SAVR), is safe and effective for treating AR.
“For Trilogy TAVI, the 30-day composite primary safety endpoint of > 40.5% was achieved,” the researchers reported in The Lancet. “2. 8% of patients who underwent Trilogy TAVI had passed at the 1-year follow-up.”
For patients with native aortic regurgitation, surgery remains the only recommended intervention. A transcatheter therapy to treat patients at high risk for complications and mortality with SAVR represents an unmet need. Commercial transcatheter heart valves in pure aortic regurgitation are hampered by unacceptable embolization and paravalvular regurgitation rates. For these patients, the Trilogy transcatheter heart valve presents a treatment option.
Torsten P Vahl, Columbia University Irving Medical Center, New York, NY, USA, and colleagues report outcomes with TAVI in patients with pure aortic regurgitation using this dedicated transcatheter heart valve.
For this purpose, the researchers conducted the ALIGN-AR trial, a prospective, multicentre, single-arm study. Symptomatic patients (aged ≥18 years) with moderate-to-severe or severe aortic regurgitation at high risk for mortality and complications after SAVI were recruited at 20 US sites for treatment with the Trilogy transcatheter heart valve.
The 30-day composite primary safety endpoint was compared for non-inferiority with a prespecified performance goal of 40·5%. The primary efficacy endpoint was 1-year all-cause mortality compared to non-inferiority with a performance goal of 25%.
346 patients were screened between 2018 and 2022. 166 patients were excluded, and 180 patients with symptomatic aortic regurgitation deemed high risk by the heart team and independent screening committee assessments, were enrolled. The patients’ mean age was 75·5 years, and 53% were male.
Based on the study, the following findings were made:
“The observed short-term hemodynamic and clinical outcomes are promising as are signs of left ventricular remodeling, but long-term follow-up is necessary,” the researchers wrote.
As advancements in technology and procedural techniques continue to evolve, TAVI holds promise as a transformative treatment modality for patients with high-risk symptomatic native aortic regurgitation. The ALIGN-AR study serves as a beacon of hope for patients and clinicians, heralding a new era in managing this complex cardiovascular condition.
Reference:
Transcatheter Aortic Valve Implantation in Patients With High-Risk Symptomatic Native Aortic Regurgitation (ALIGN-AR): A Prospective, Multicenter, Single-Arm Study. Lancet 2024; Mar 26:[Epub ahead of print].
Powered by WPeMatico
China: The correlation between COVID-19 infection severity and pre-existing health conditions has been a focal point of scientific inquiry since the onset of the pandemic. Among the various comorbidities, diabetes has emerged as a significant risk factor, exacerbating the severity of the viral illness, according to a recent study published in the Journal of Health, Population and Nutrition.
The researchers reported that CT imaging reveals the influence of blood glucose levels on the severity of COVID-19.
The study revealed a significant correlation between blood glucose control and CT severity score (CTSS); the higher the blood glucose is, the more severe the lung manifestation. Also, the CTSS can be used to predict and evaluate COVID-19’s clinical severity.
Previous studies have shown that patients with diabetes exhibit more severe conditions concerning the clinical course and chest imaging and are more likely to develop critical COVID-19 compared with normal-infected individuals. Furthermore, those with poor blood glucose control have a worse prognosis.
Against the above background, Jin Wang from the Yan’an Hospital Affiliated with Kunming Medical University in Kunming, Yunnan, China, and colleagues aimed to analyze the correlation between blood glucose control and the severity of COVID-19 infection in diabetes patients.
For this purpose, the researchers retrospectively collected imaging and clinical data of 146 patients with diabetes combined with COVID-19 who visited the hospital between 2022 and 2023.
The patients were categorized into two groups based on an assessment of their blood glucose control: the good blood glucose control group and the poor blood glucose control group. The two groups were compared for the clinical data, computed tomography (CT) appearance and score, and the severity of COVID-19 infection, with the COVID-19 severity being the dependent variable to analyze other influencing factors.
The study led to the following findings:
· The group with poor blood glucose control showed a higher lobar involvement degree and total CT severity score than the group with good blood glucose control (13.30 ± 5.25 versus 10.38 ± 4.84).
· The two groups exhibited no statistically significant differences in blood lymphocyte, leukocyte, pleural effusion, C-reaction protein, consolidation, ground glass opacity, or crazy-paving signs.
· Logistic regression analysis showed that the total CTSS significantly influences the clinical severity of patients (odds ratio 1.585). In contrast, fasting plasma glucose and blood glucose control are not independent factors influencing clinical severity.
· The area under the curve (AUC) of CTSS prediction of critical COVID-19 was 0.895 with sensitivity of 79.3% and specificity of 88.1% when the threshold value is 12.
In conclusion, chest CT imaging is a reliable testing method for evaluating COVID-19; the higher the blood glucose level is, the more severe the clinical manifestation and the more evident the lung inflammation. This indicates that clinical patients should receive a CT scan upon admission to evaluate their initial condition, and there should be a thorough analysis of imaging appearances.
“Targeted treatment should be administered, and education and publicity activities should be implemented aiming to popularise the necessity of controlling their blood glucose among patients with diabetes and encouraging them to make an active effort to keep healthy and follow appropriate diets, thus improving their quality of life,” the researchers wrote.
Reference:
Lu, D., Liu, Y., Ma, P. et al. Severity of COVID-19 infection in patients with COVID-19 combined with diabetes. J Health Popul Nutr 43, 55 (2024). https://doi.org/10.1186/s41043-024-00548-w
Powered by WPeMatico

Mediterranean diet linked to improved pain and quality of life in fibromyalgia patients suggests a study published in Pain Therapy.
Fibromyalgia is a form of chronic pain that affects a large number of women. It can start at any age and last a lifetime, with no cure. The Mediterranean diet is said to have an anti-inflammatory effect. Therefore, this study was conducted to investigate the possible beneficial effects of a personalized Mediterranean diet in patients with fibromyalgia. Outpatients with fibromyalgia were recruited and invited to participate in the study, including clinical, nutritional, and dietary assessments. Patients received a personalized Mediterranean diet (DIET group) or a generally balanced diet (NODIET group) to be followed for 8 weeks. All tests were carried out at baseline and repeated after 4 and 8 weeks. Results: In total, 100 subjects were included, 84 of whom completed the study. Most of the patients showed incorrect habits in terms of food choices, timing of meals and composition of nutrients. The DIET group showed an improvement in most of the fibromyalgia parameters, including disability scores, fatigue, and anxiety. The habit of eating inflammatory foods and/or eating meals with the wrong nutritional content would increase the negative status of patients with fibromyalgia. With this study, we confirm that proper attention to feeding habits would improve the quality of life of such patients.
Reference:
Casini, I., Ladisa, V., Clemente, L. et al. A Personalized Mediterranean Diet Improves Pain and Quality of Life in Patients with Fibromyalgia. Pain Ther (2024). https://doi.org/10.1007/s40122-024-00598-2
Keywords:
Mediterranean diet, improved, pain, quality of life, fibromyalgia, patients, Casini, I., Ladisa, V., Clemente, L, Pain therapy
Powered by WPeMatico

USA: A recent study published in the American Journal of Preventive Cardiology has suggested the benefits of aspirin in primary prevention could be dependent on lipoprotein(a) [Lp(a)] levels of a person.
The study found a significantly lower atherosclerotic cardiovascular disease (ASCVD) mortality in adults without clinical ASCVD with elevated Lp(a). Given the high prevalence of predominantly genetically determined elevated Lp(a), the findings may have public and clinical health implications for aspirin utilization in primary prevention.
Analyzing more than 2900 people with a median follow-up of 26 years, the researchers found that regular aspirin use in those aged 40 to 70 years cut the risk of ASCVD mortality in half among those with Lp(a) levels of 50 mg/dL or more but had no benefit among those with reduced Lp(a).
Lipoprotein(a) is an atherogenic and prothrombotic lipoprotein associated with ASCVD. Alexander C. Razavi, Emory Clinical Cardiovascular Research Institute, Emory University School of Medicine, Atlanta, GA, and colleagues aimed to assess the association between regular aspirin use and ASCVD mortality among individuals with versus without elevated Lp(a) in a nationally representative US cohort.
Eligible participants were aged 40–70 years without clinical ASCVD, reported on aspirin use, and had Lp(a) measurements from the Third National Health and Nutrition Examination Survey (NHANES III, 1988–1994), the only cycle of this nationally representative US cohort to measure Lp(a).
Regular aspirin use was defined as aspirin intake ≥30 times in the previous month. Over a median 26-year follow-up, using NHANES III linked mortality records and weighted Cox proportional hazards regression, the association between regular use of aspirin and ASCVD mortality was observed in those with and without elevated Lp(a) (≥50 versus <50 mg/dL).
The study revealed the following findings:
“Regular aspirin use was associated with a 52% lower risk of ASCVD mortality among adults without clinical ASCVD who had elevated Lp(a),” the researchers wrote. “These findings suggest that Lp(a) measurement may help identify individuals who benefit from aspirin therapy for primary prevention.”
Reference:
Razavi, A. C., Richardson, L. C., Coronado, F., Dzaye, O., Bhatia, H. S., Mehta, A., Quyyumi, A. A., Vaccarino, V., Budoff, M. J., Nasir, K., Tsimikas, S., Whelton, S. P., Blaha, M. J., Blumenthal, R. S., & Sperling, L. S. (2024). Aspirin Use for Primary Prevention Among US Adults With and Without Elevated Lipoprotein(a). American Journal of Preventive Cardiology, 100674. https://doi.org/10.1016/j.ajpc.2024.100674
Powered by WPeMatico
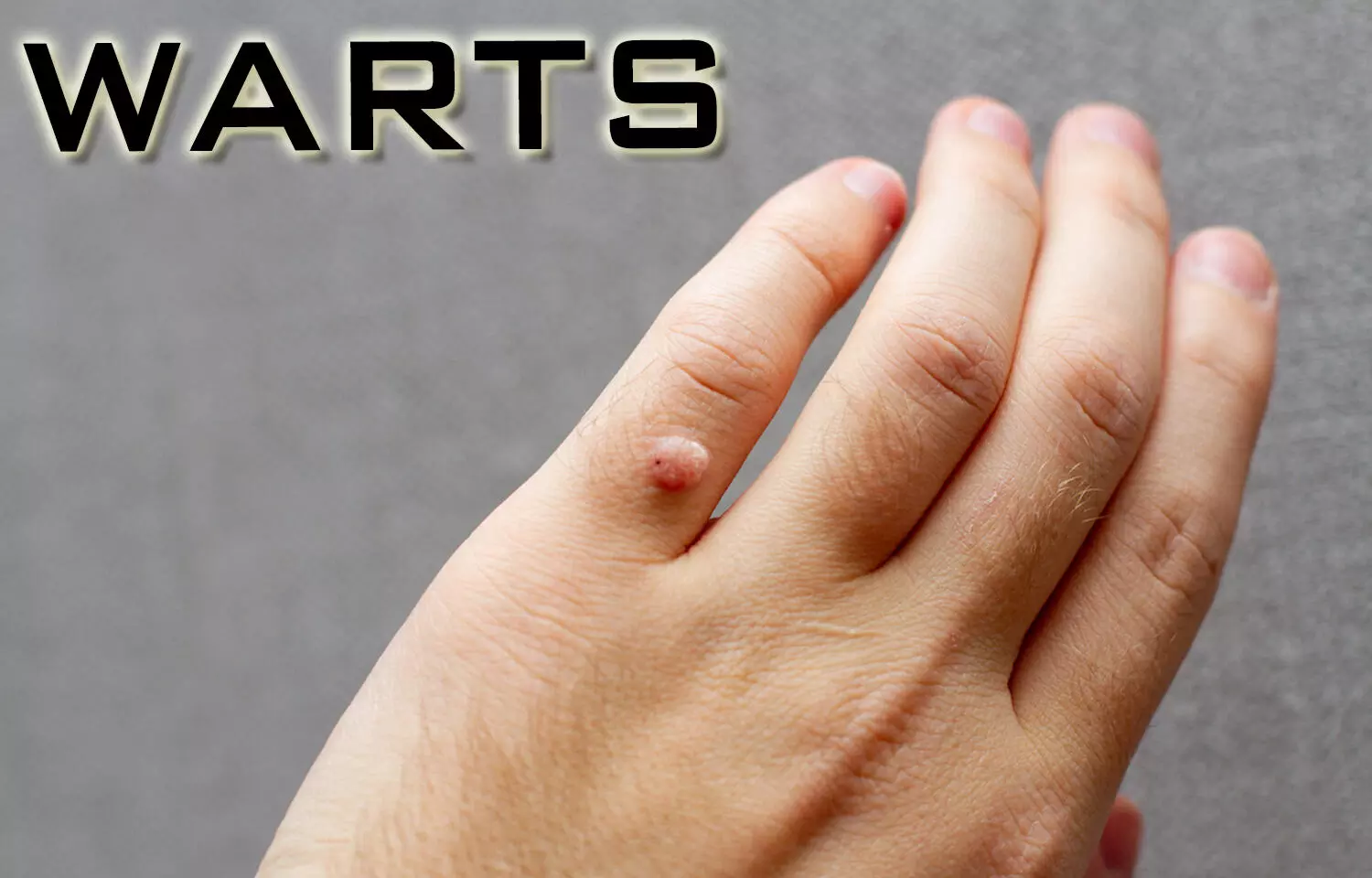
Researchers have found that tazarotene 0.1% gel and imiquimod 5% cream offer similar efficacy in treating plane warts, according to a three-arm randomized controlled trial. Both treatments demonstrated significant improvements compared to baseline and placebo, but tazarotene may offer additional advantages, such as a safer profile regarding dyspigmentation and cost savings. The study was published in the journal Clinical and Experimental Dermatology by Hagar N. and colleagues.
Plane warts, particularly when multiple and recurrent, can be challenging to treat and may negatively impact a patient’s quality of life. For lesions located in cosmetically sensitive areas, topical treatments are often preferred to minimize potential sequelae. This study aimed to compare the efficacy and tolerability of tazarotene 0.1% gel and imiquimod 5% cream in treating plane warts.
In this parallel three-arm randomized controlled trial, 60 patients were assigned to one of three groups: imiquimod, tazarotene, or placebo. Patients applied the corresponding treatment once daily at night for up to 12 weeks. The primary outcomes measured were the percentage of respondents with complete clearance and the type and frequency of side effects in each group.
The key findings of the study were as follows:
Complete clearance was achieved in 50% of patients, while 15% experienced partial response and 35% showed no response.
Complete clearance was achieved in 40% of patients, with another 40% experiencing partial response and 20% showing no response.
There was no significant difference in treatment efficacy between the imiquimod and tazarotene groups (P=0.190).
Both active treatments provided significant improvement over baseline and the placebo group (P=0.001).
However, tazarotene 0.1% gel may offer advantages such as a safer profile regarding dyspigmentation and a lower cost.
Both treatments were generally well-tolerated, with no significant safety concerns reported. Side effects, including local irritation and inflammation, were manageable and did not differ significantly between the groups.
Tazarotene 0.1% gel appears to offer equivalent efficacy to imiquimod 5% cream in treating plane warts. Moreover, it provides maintained efficacy without recurrence, a safer profile regarding dyspigmentation, and an advantageous cost benefit. These findings suggest that tazarotene could be a preferred option for treating plane warts, particularly in cosmetically significant areas.
Reference:
Nofal, H., Omran, F., ElKholy, B., Nofal, S., & Nofal, A. (2024). Tazarotene is as effective and tolerable as imiquimod in the treatment of verruca plana, a comparative randomized clinical trial. Clinical and Experimental Dermatology. https://doi.org/10.1093/ced/llae133
Powered by WPeMatico
