How an Alzheimer’s peptide and a blood protein may combine to drive early disease pathology
Powered by WPeMatico
Powered by WPeMatico

New Delhi: Prime Minister Shri Narendra Modi extended warm greetings to people across India and the world on the occasion of the 11th International Day of Yoga.
The Prime Minister led the Yoga Day celebrations and took part in a Yoga session at an event in Vishakhapatnam, in Andhra Pradesh, today. He said, “Eleven years on, yoga has become an integral part of the lifestyle of millions across the globe.”
Prime Minister Narendra Modi expressed his deep satisfaction in witnessing the visually impaired (Divyang) reading yogic scriptures in Braille and applauded scientists practicing yoga even in outer space. He highlighted the active involvement of youth from rural areas in the Yoga Olympiads, emphasizing that from iconic landmarks like the Sydney Opera House to the peak of Mount Everest and the vast expanse of the ocean, the consistent message is: “Yoga is for everyone and for all, Beyond Boundaries, Beyond Backgrounds, Beyond age or ability.”
He noted that over two crore people have joined the Yogandhra Abhiyan, which he described as a vibrant display of public engagement. Prime Minister stressed that such enthusiastic citizen participation forms the cornerstone of a Viksit Bharat. When people take initiative and ownership of a cause, he asserted, achieving any goal becomes possible.
Also Read:AYUSH Ministry hosts 3-day Yoga Maha Kumbh in Delhi
Speaking about this year’s International Day of Yoga theme, “Yoga for One Earth, One Health”, Prime Minister said it conveys a fundamental truth: the well-being of all life on Earth is interconnected. He explained that human health is tied to the quality of soil, the purity of water, the well-being of animals, and the nourishment we receive from plants.
He said yoga helps awaken us to this deep connection and guides us toward unity with the world. “Yoga teaches us we are not isolated individuals but integral parts of nature. Initially, we learn to care for our own health and wellness, but gradually, this care expands to our environment, society, and the planet. Yoga is a profound personal discipline that, at the same time, serves as a collective system – one that transitions individuals from Me to We,” he stated.
He continued by saying that, “The spirit of ‘Me to We’ encapsulates the very soul of India,” and reflected on Indian values that encourage individuals to rise above personal interest and embrace the welfare of all. Referring to the cultural ethos, he said that India teaches the value of “Sarve Bhavantu Sukhinah” – the happiness and well-being of all is a sacred responsibility. This philosophy, he added, lays the foundation for service, selflessness, and harmony.
The Prime Minister expressed his concern about rising levels of stress, instability, and conflict across the world. He pointed to yoga as a solution, stating that “Yoga is the pause button humanity needs, to breathe, to balance, to become whole again.” He made a heartfelt appeal to the global community, proposing that “this Yoga Day mark the beginning of Yoga for Humanity 2.0, where Inner Peace becomes Global Policy.”
He emphasized the importance of making yoga more than just a personal routine—it should become a catalyst for global partnerships. Shri Modi called on all countries and societies to embed yoga into daily life and public policy, expressing his vision for a united effort to build a more peaceful, balanced, and sustainable planet. “Yoga should guide the world from conflict to cooperation, and from stress to solutions,” he concluded.
Raising awareness about the global challenge of obesity, the Prime Minister revisited his previous Mann Ki Baat address, where he had launched a campaign to reduce daily oil consumption by 10 percent. He renewed his appeal for people across India and the world to participate in this initiative. Shri Modi underscored that reducing oil intake, avoiding harmful dietary habits, and integrating yoga into daily life are essential for better health.
In his closing remarks, the Prime Minister urged everyone to make yoga a jan andolan. He envisioned a global wave of peace, wellness, and harmony driven by the power of yoga. He encouraged individuals to begin each day with yoga for personal balance and encouraged societies to embrace yoga to alleviate collective stress. Ending on a hopeful note, he affirmed: “Yoga should serve as a thread that weaves humanity together, Yoga for One Earth, One Health should become a global resolution.”
Union Minister of Health and Family Welfare, Shri Jagat Prakash Nadda marked the 11th International Yoga Day celebrations by leading a mass yoga demonstration with hundreds of participants in Kartavya Path, New Delhi.
Speaking on the occasion, Shri Nadda stated that, “It is due to Prime Minister Shri Narendra Modi’s efforts that Yoga Day is being celebrated internationally today. During his address to the UN General Assembly in 2014, Hon’ble Prime Minister had proposed for Yoga to be celebrated internationally every year on 21st June, which was backed by over 170 countries.”
Shri Nadda added that, “since 2015, yoga has found global acceptance as a holistic approach to well-being. Yoga not only improves people’s health and fitness but also enhances mental clarity and emotional regulation, contributing to more balanced and fulfilling life.”
The Union Health Minister informed that the 11th International Day of Yoga will witness crores of participants performing yoga from lakhs of destinations across India. He said, “It is a moment of pride that we all are gathered here to celebrate this ancient Indian knowledge system, uplift our body and mind in the process and understand ourselves better through the gift of Yoga.”
Shri Nadda highlighted that yoga has been included under the National Health Policy 2017 to offer a more holistic and accessible approach to healthcare. He also pointed out a new Centre for Integrative Medicine and Research has been set up in AIIMS Delhi to provide integrative solutions encompassing traditional medical systems like Yoga and modern medicines to address health concerns.
He concluded his address by encouraging all participants to make Yoga a part of their daily life and work towards a healthy nation.
Union Minister of State for Health and Family Welfare, Shri Prataprao Jadhav stated that “Yoga is a celebration of the universal consciousness that comes from the soul of India and has not only touched millions of lives but has also paved a lasting path for global health and collective peace”.
He also highlighted that, “In the last ten years, India, under the leadership of the Prime Minister, has established yoga not just as an ‘exercise’ or ‘alternative medicine’ but as a way of life. This is the era of moving forward by combining the spiritual depth, scientific authenticity and social harmony of yoga.”
Smt. Anupriya Singh Patel, Union Minister of State for Health and Family Welfare, noted that “Yoga is an invaluable gift of Indian culture, which has given the whole world the direction to live a healthy life”. “This is a wonderful science that connects body, mind and soul”, she stated.
Also Read:AYUSH Ministry Kicks Off 11th Yoga Day celebrations, PM Modi to lead Mega Event
Powered by WPeMatico

Panaji: To enhance the healthcare services on par with international standards, the Goa government has decided to constitute an advisory board of experts from the All India Institute of Medical Sciences (AIIMS), the World Health Organisation (WHO), the Union Government, and specialists from across the country. This board will monitor the functioning of the Goa Medical College and Hospital (GMCH).
Located at Bambolim near Panaji, the GMCH is Goa’s largest government-run hospital with more than 1,000 beds, and caters to patients from adjoining regions of Maharashtra and Karnataka. In response to increasing patient footfall at GMCH, the government aims to upgrade the hospital’s services and overall functionality, striving to bring it up to the standards of international healthcare systems.
Also read- Massive Uproar after Goa Health Minister Berates, Suspends Medical Officer, Doctors Demand Apology
Talking to PTI, state Health Minister Vishwajit Rane said the board will be headed by him with representatives from All India Institute of Medical Sciences (AIIMS), World Health Organisation (WHO) India, the Union government and top doctors across the country. The board members will guide to ensure that the services at the apex hospital are on par with international standards, he said. He said that the board will have the state government’s Additional Secretary (Health) as its member secretary.
This comes after the recent controversy at Goa Medical College and Hospital (GMCH), where the Health Minister publicly berated a senior doctor and ordered his suspension, alleging that the doctor had refused to treat a senior citizen and had behaved inappropriately.
As reported by Medical Dialogues, the incident sparked outrage within the medical community, with many demanding an apology from the Minister for the public humiliation of the doctor. However, Minister Rane stood by his actions, refusing to apologise and asserting that he acted in defence of a helpless patient and would continue to raise his voice against any form of patient discrimination.
Following the incident, the government has decided to uplift the quality of services at GMCH by forming an advisory board of experts who will provide guidance and recommendations to improve the hospital’s functioning.
Rane said that an advisory board for the GMCH existed in the past. Speaking to The Hindu, he said, “The advisory board for the GMCH existed in the past when I was the Health Minister, but when the next Minister came in, he dismantled it. Back then, we also had medical experts from AIIMS, the WHO, and a couple of doctors from Mumbai. This advisory board is absolutely crucial, with independent experts onboard, along with the other government agencies. The board will also decide the standard operating procedures for specialised treatments in the hospital.”
“We want to increase the efficiency of the hospital and provide good care to the patients with the ultimate objective of helping the poor and needy people. The advisory board will further strengthen the vision of Prime Minister Narendra Modi of providing international standard health care in all the states,” Rane added.
The specialised doctors who will be part of the advisory board include a team of around 12 to 13 experts from across the country, including cities such as Mumbai, Manipal, Delhi, Bhopal, and Arunachal Pradesh. However, the final list of board members has not yet been confirmed and is still in the process of being finalised.
“So far, we have Dr. R. P. Srivastava, past president, the Association of Surgeons of India; Dr. Ajai Singh from AIIMS Bhopal; and Dr. Sagar Galwankar, emergency medicine specialist associated with AIIMS. From Mumbai, we will have Dr. Ashok Johari, a renowned orthopaedic surgeon; Padmashri Dr. Amit Maydeo, Chairman of the Institute of Gastrosciences, Sir HN Reliance Foundation Hospital; and Dr. Sunil Bandekar from the Breach Candy Hospital. Once we have the team, we will divide them into two groups and have a quarterly in-person meeting and a monthly virtual meeting,” Mr. Rane told the Daily.
According to him, qualified doctors would be part of the specialised medical care system, while others would be sent for training.
Also read- Goa Medical College Gets Approval to Increase 19 seats in 5 MD, MS specialities
Powered by WPeMatico

Erode: A doctor at the Primary Health Centre (PHC) in Siruvalur has come under scrutiny after allegedly telling a pregnant woman that she was not pregnant and attributing her abdominal swelling to bloating.
The woman later underwent a scan at a private hospital, which confirmed that she was four months pregnant. This discrepancy has led to serious allegations of medical negligence.
According to the news reports, the 28-year-old woman had been receiving regular prenatal care at the Primary Health Centre (PHC) in Ayalur, which falls under the jurisdiction of the Siruvalur Block PHC in Gobichettipalayam. As part of routine procedures to monitor foetal development, she was referred to the Siruvalur PHC for a follow-up evaluation.
Also Read: Misdiagnosed for years, Elderly man’s life saved by rare tumour surgery at KGMU
A few days ago, during her visit to the Siruvalur centre, a trainee doctor examined the woman and informed her that she was not pregnant, claiming the abdominal swelling was due to bloating. Shocked and confused, the couple sought a second opinion at a private hospital in Gobichettipalayam, where an ultrasound scan confirmed that she was four months pregnant and the fetus was developing normally.
The couple has since raised allegations of medical negligence against the doctor at the Siruvalur PHC, saying their repeated efforts to meet or speak with the medical officer in question were unsuccessful. They have demanded that appropriate action be taken by the health authorities.
Speaking to the Hindu, Deputy Director of Health Services P. Aruna said that the doctor had cited poor abdominal visibility during the scan as the reason for the confusion. She added that an inquiry was underway.
Also Read: Misdiagnosed Fetal Deformity: Scan Centre slapped Rs 16.5 Lakh compensation
Powered by WPeMatico

Guwahati: Assam Chief Minister Himanta Biswa Sarma on Wednesday distributed appointment letters to 400 health officers, asserting that his government is committed to creating employment opportunities for youths.
At an official function here, Sarma distributed the appointment letters among ‘Medical and Health Officers’, and said it marked a cumulative total of 1,20,359 recruitments provided to youths in the state.
“Taking a leap towards our goal of over 1.5 lakh government jobs, I am distributing appointment letters to 400 Medical and Health Officers. These officers will add muscle to our efforts of delivering quality healthcare to the last mile at the grassroots,” he said, news agency PTI reported.
Also Read:CM Dhami distributes appointment letters to 1,232 nursing officers
In the last few years, delivering quality healthcare and providing jobs have been the key priorities of the Assam government, Sarma said.
“With these appointments, the aspirations of millions of people of getting better healthcare delivery and services will be fulfilled,” he said.
The CM also said the state government’s robust interventions in the healthcare sector in the last few years have ensured that there has been a rapid reduction in maternal mortality rate and infant mortality rate.
“In recent years, Assam has seen a sharp decline in maternal mortality rate. From 480 when I first took charge as health minister to 167 now, the numbers will plunge more. With continued efforts, Assam is expected to reach the national average in the next 3-4 years. Our efforts in this direction will continue,” he said.
The Assam government’s goal is to have 30 medical colleges by 2029, and it is moving ahead in this direction in a systematic manner, Sarma said.
“From just three in 2006, today we have 13 functional medical colleges, delivering quality healthcare at affordable rates. The results have been gratifying… healthy citizens, finest treatment and also many additional employment opportunities for our youth.
“When I initiated work on Jorhat Medical College as health minister, people questioned my decision and criticised every step taken, but we still succeeded. Today, the inauguration of a medical college has become a routine affair,” he said.
The chief minister also said the private sector should complement public health institutions, not compete with it for patients.
“Only then will our medical landscape evolve into a patient-centric one with clear roles set for public and private health institutions,” he added.
Also Read:GMCH set to become one of India’s largest hospitals, says Assam CM
Powered by WPeMatico
USA: Frailty is common among older patients undergoing percutaneous coronary intervention (PCI) in the United States, with greater frailty linked to significantly higher risks of in-hospital death and procedural complications, according to new findings from the American College of Cardiology’s CathPCI Registry. The study, led by Dr. Benjamin Peterson from the University of Kentucky School of Medicine, was published online in JACC ahead of its June 24, 2025, issue.
The analysis included over 1.3 million patients (mean age 74.6 years; 34% women) aged 65 years or older who underwent PCI between October 2018 and December 2021. Frailty was evaluated using the Canadian Study of Health and Aging Clinical Frailty Scale (CFS), which assesses the ability to perform both basic and instrumental activities of daily living. Patients were classified into four groups: non-frail (CFS 1-2), prefrail (CFS 3-4), frail (CFS 5-6), and severely frail (CFS 7-9).
The key findings were as follows:
While the authors caution against using these findings to withhold PCI, especially in acute coronary syndromes, they emphasize the importance of incorporating frailty assessment into routine clinical evaluations. “Frailty status, even at the prefrail level, significantly affects procedural risk and should guide shared decision-making,” the authors noted.
In an accompanying editorial, Dr. John A. Dodson and Dr. Ashok Krishnaswami advocate for incorporating frailty screening into routine patient assessment, similar to vital signs, to enhance outcomes for older adults undergoing PCI.
“To meaningfully improve care for older adults, frailty assessment should become a routine part of clinical practice—integrated into every evaluation just as vital signs are,” they wrote.
Reference:
Peterson, B., Kochar, A., Young, R., Senman, B., Rymer, J., Wojdyla, D., … Bhatt, D. L. (2025). Effect of Frailty on In-Hospital Mortality and Complications of PCI: An NCDR Registry Report (Accepted). Journal of the American College of Cardiology, 85(24), 2416–2420. https://doi.org/10.1016/j.jacc.2025.04.027
Powered by WPeMatico
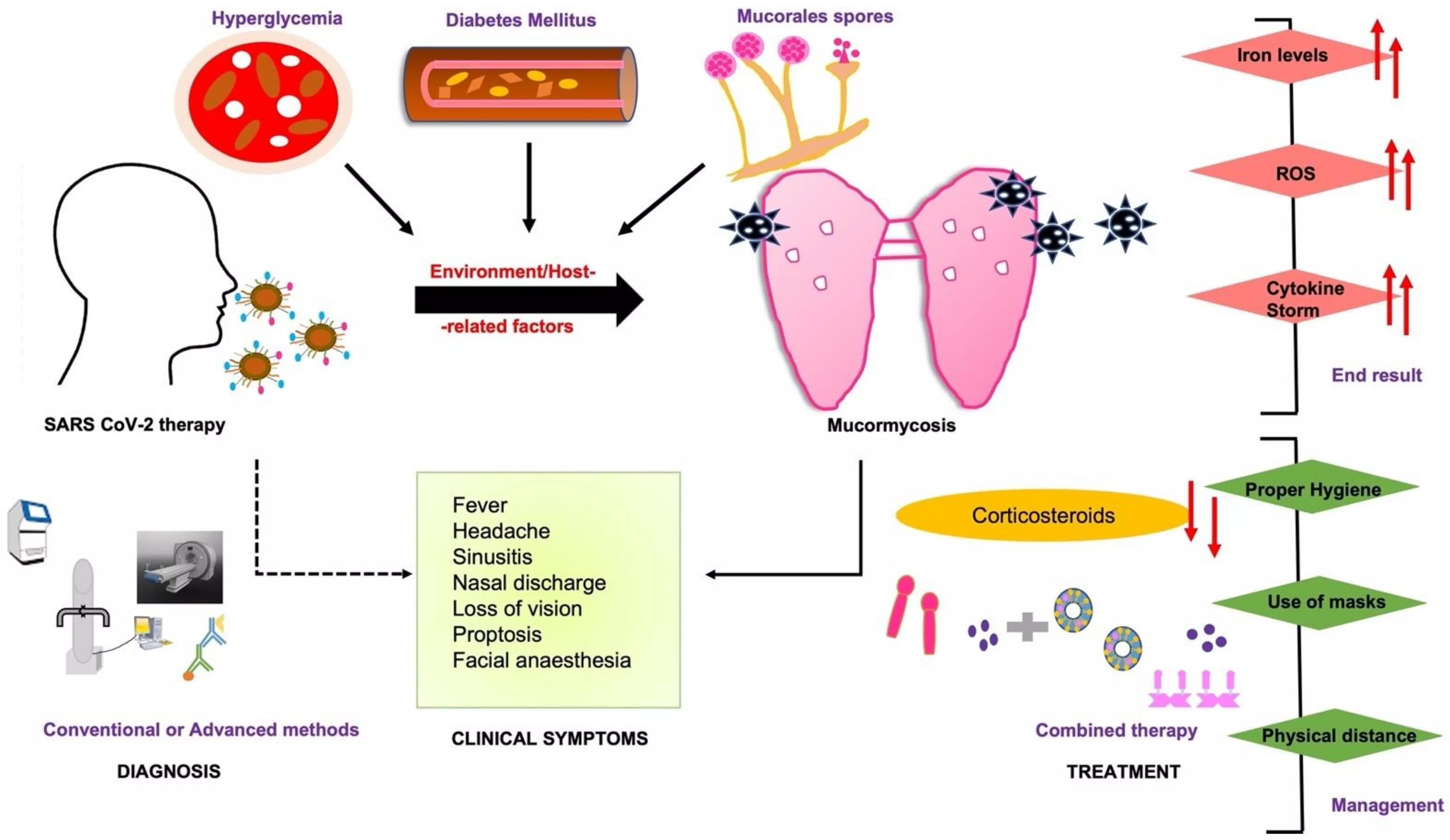
Researchers have found in a new research that the combined use of oral posaconazole, adjunctive hyperbaric oxygen (HBO) therapy, and methylene blue effectively prevents further progression of COVID-19 associated sino-maxillary and mandibular mucormycosis.
The study aimed to assess the cumulative role of oral posaconazole, post-debridement Hyperbaric Oxygen (HBO) therapy, along with local application and steam inhalation with methylene blue as an adjunct to surgery to prevent further progression of COVID-19 associated sino-maxillary or mandibular mucormycosis.
A retrospective observational study was conducted on patients diagnosed with sino-maxillary or mandibular mucormycosis associated with COVID-19. A total of 38 patients underwent surgical debridement and received adjunctive treatment with posaconazole, hyperbaric oxygen therapy, and local application and steam inhalation of methylene blue. Medical records were analyzed for COVID-19-related hyperglycemia, prolonged hospitalization, immunosuppressive/steroid therapy, and the use of iron or zinc supplements, which may be linked to increased mucormycosis incidence. Outcomes were evaluated for association with any concurrent infection, complications, need for sequential debridement, and further progression of disease.
Results: A total of 38 patients (range: 23–68) were included, comprising 24 males and 14 females. Isolated sino-maxillary involvement was observed in 32 cases (84.21%), and mandibular involvement in 5 cases (13.15%). No further disease progression was noted during the 2-year follow-up, based on clinical evaluation and postoperative computed tomography (CT) scans. Actinomycosis co-infection was identified in 21.05% of cases.
Complications included wound dehiscence (39.47%), pus discharge (5.26%), reversible hearing impairment during HBO therapy (5.26%), and flap necrosis (2.63%). Four patients (10.52%) required sequential surgical debridement for sequestrum removal. It was concluded that concurrent use of oral posaconazole, adjunct HBO therapy and methylene blue prevents further progression of COVID-19 associated sino-maxillary and mandibular mucormycosis.
Reference:
Mohanty, S., Bansal, N., Verma, A. et al. Adjunctive use of posaconazole, hyperbaric oxygen therapy, and methylene blue for COVID-19-associated mucormycosis. Oral Maxillofac Surg 29, 126 (2025). https://doi.org/10.1007/s10006-025-01419-2
Powered by WPeMatico
Phase 3 trials have demonstrated that enlicitide decanoate significantly reduces LDL cholesterol, showing strong potential as an oral PCSK9 inhibitor for patients with hyperlipidemia and heterozygous familial hypercholesterolemia (HeFH).
The CORALreef HeFH and CORALreef AddOn trials successfully met their primary and all key secondary endpoints, demonstrating statistically significant and clinically meaningful greater reductions in low-density lipoprotein cholesterol (LDL-C) for enlicitide compared to placebo (CORALreef HeFH) and compared to other oral non-statin therapies (CORALreef AddOn). There were no clinically meaningful differences in incidences of adverse events (AE) and serious adverse events (SAE) in either trial.
Results from the three Phase 3 trials in the CORALreef clinical development program will be presented at a future scientific congress.
• CORALreef HeFH: statistically significant and clinically meaningful reductions in LDL-C for enlicitide versus placebo in adults with heterozygous familial hypercholesterolemia (HeFH) who have a history of or are at risk for atherosclerotic cardiovascular disease (ASCVD) and are treated with a statin.
• CORALreef AddOn: statistically significant and clinically meaningful reductions in LDL-C for enlicitide versus ezetimibe, versus bempedoic acid and versus ezetimibe and bempedoic acid in adults with hyperlipidemia who have a history of or are at risk for ASCVD and are treated with a statin.
“We are thrilled to bring forward the first Phase 3 results from our clinical development program evaluating enlicitide, which, if approved, would be the first marketed oral PCSK9 inhibitor in the U.S.,” said Dr. Dean Y. Li, president, Merck Research Laboratories. “Enlicitide is a novel macrocyclic peptide that has the potential to deliver antibody-like efficacy and specificity for the validated PCSK9 mechanism in the form of a daily oral pill. We are working with urgency to make this oral therapy available to patients worldwide.”
“Atherosclerotic cardiovascular disease accounts for 85 percent of cardiovascular deaths. Despite available treatment options, cardiovascular-related deaths remain the leading cause of death worldwide and continue to rise,” said Dr. Christie M. Ballantyne, principal investigator of the CORALreef HeFH study and Professor of Medicine at Baylor College of Medicine. “LDL-C is a major modifiable risk driver for atherosclerosis and prioritization of LDL-C management should be a cornerstone of cardiovascular risk prevention. Early intervention and intensification of lipid treatment would allow more patients to achieve LDL-C goals.”
The efficacy and safety of enlicitide are being evaluated through the comprehensive CORALreef Phase 3 clinical development program, which aims to enroll approximately 17,000 patients across several trials, including two large ongoing trials, CORALreef Lipids and CORALreef Outcomes.
CORALreef HeFH (NCT05952869) is a Phase 3, randomized, double-blind, placebo-controlled, multicenter study designed to evaluate the safety and efficacy of enlicitide compared to placebo in adults with HeFH who have a history of or were at risk for a major ASCVD event and are treated with a moderate or high intensity statin with or without other lipid-lowering therapies. The primary endpoints were mean percent change from baseline in LDL-C at week 24, number of participants with one or more adverse events (AEs), and number of participants who discontinued study drug due to an AE. Secondary endpoints included mean percent change from baseline in LDL-C at week 52, mean percent change from baseline in non-HDL-C, ApoB and percent change in Lp(a) at week 24.
CORALreef AddOn (NCT06450366) is a Phase 3, randomized, double-blind, multicenter study designed to evaluate the efficacy and safety of enlicitide compared to ezetimibe, to bempedoic acid, and to ezetimibe and bempedoic acid, in patients with hypercholesterolemia who had a history of a major ASCVD event or were at risk for a major ASCVD event and are treated with a statin. The primary endpoint was the mean percent change from baseline in LDL-C at week 8. Secondary endpoints included mean percent change from baseline in non-HDL-C and ApoB.
Enlicitide is an investigational, potentially first oral PCSK9 inhibitor designed to lower LDL-C via the same biological mechanism as currently approved monoclonal antibody injectable PCSK9 inhibitors but in a daily pill form. Enlicitide is a novel oral macrocyclic peptide that binds to PCSK9 and inhibits the interaction of PCSK9 with LDL receptors.
PCSK9 plays a key role in cholesterol homeostasis by regulating levels of the LDL receptor, which is responsible for the uptake of cholesterol into cells. Inhibition of PCSK9 with enlicitide prevents the interaction of PCSK9 with LDL receptors. This results in greater numbers of LDL receptors available on the cell surface to remove LDL cholesterol from the blood.
Hyperlipidemia is a disorder characterized by an excess of lipids or fats in the blood, affecting approximately 86 million adults (aged 20 and older) in the U.S. Despite adjusting diet or other lifestyle factors, some individuals may not reach recommended lipid levels and will require medication to treat and manage hyperlipidemia. Hyperlipidemia is a major risk driver for the development of ASCVD events, such as heart attacks and strokes, which account for 85 percent of cardiovascular deaths.
Powered by WPeMatico
New Delhi: In response to the proposal presented by Zydus Healthcare, the Subject Expert Committee (SEC) functional under the Central Drug Standard Control Organisation (CDSCO) has recommended the drug maker to conduct the Phase IV clinical trial of the fixed dose combination (FDC) Indacaterol maleate plus Budesonide Powder for inhalation (capsules).
In addition, the committee recommended the firm to submit the Phase IV clinical trial report of the FDC Indacaterol maleate plus Budesonide Powder for inhalation.
This came after the firm presented the Phase IV clinical trial protocol before the committee.
Indacaterol is a long-acting β2-adrenoceptor agonist and bronchodilator with a rapid onset of action. It was developed by Novartis. It is used to relax bronchial smooth muscle and improve symptoms and airflow obstruction caused by Chronic Obstructive Pulmonary Disease (COPD) and moderate to severe asthma.
Budesonide is a glucocorticoid that acts as an anti-inflammatory and immunomodulator. Budesonide inhalation is a treatment for asthma and chronic obstructive pulmonary disease (COPD).
At the recent SEC meeting, the expert panel reviewed the Phase IV clinical trial protocol before the committee.
After detailed deliberation, the committee recommended for grant of permission to conduct of the Phase IV clinical trial. “Accordingly, the firm should submit the Phase IV clinical trial report to CDSCO for further review by the committee,” the Panel noted.
Powered by WPeMatico

Canada: A comprehensive meta-analysis has found that patient-facing digital inhalers may significantly improve asthma management, particularly among individuals who struggle with medication adherence or inhalation techniques. The findings, published in The Journal of Allergy and Clinical Immunology: In Practice, indicate that these devices likely enhance asthma control and could lower the risk of severe asthma exacerbations with minimal associated harm.
Digital inhalers have sensors that provide real-time feedback to patients regarding their medication usage and inhalation technique. These tools aim to address the long-standing issue of poor adherence, estimated to affect around 43% of asthma patients globally.
To better understand their clinical utility, Leonardo Ologundudu, from McMaster University in Ontario, Canada, and colleagues conducted a systematic review of 12 randomized controlled trials involving 2,483 children (ages 4–17) and adults. This work was undertaken in the context of developing new clinical guidelines for severe and difficult-to-control asthma by the American Academy of Allergy, Asthma & Immunology and the American College of Allergy, Asthma, and Immunology Joint Task Force.
Based on the study, the researchers reported the following findings:
The authors emphasize considering the benefits of digital inhalers within a broader clinical context. Patients already demonstrating consistent adherence and proper technique may derive limited additional benefit, whereas those requiring ongoing support might find digital inhalers especially helpful.
However, limitations exist. Many studies lacked blinded outcome assessors, and few enrolled older adults above 60. Moreover, the combined effect of digital inhalers and remote clinician monitoring was commonly studied, making it difficult to isolate its standalone impact.
Still, the findings are expected to influence future clinical practice. “These results support the incorporation of digital inhalers into asthma management, particularly for select patient subgroups. Updated guidelines should reflect this evolving landscape,” the researchers concluded.
Reference:
Ologundudu L, Rayner DG, Oppenheimer J, Sumino K, Hoyte F, RiveraSpoljaric K, Perry TT, Nyenhuis SM, Chipps B, Israel E, Shade LE, Press VG, Rangel S, Guyatt GH, McCabe E, O’Byrne PM, Hall L, Orr H, Sue-Wah-Sing D, Melendez A, Winders T, Przywara K, Gardner DD, Rank MA, Bacharier LB, Mosnaim G, Chu DK, Patient-facing digital inhalers for asthma: a systematic review and meta-analysis, The Journal of Allergy and Clinical Immunology: In Practice (2025), doi: https://doi.org/10.1016/j.jaip.2025.04.039.
Powered by WPeMatico
