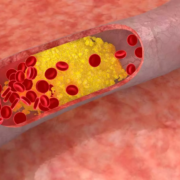
Phase 3 trials have demonstrated that enlicitide decanoate significantly reduces LDL cholesterol, showing strong potential as an oral PCSK9 inhibitor for patients with hyperlipidemia and heterozygous familial hypercholesterolemia (HeFH).
The CORALreef HeFH and CORALreef AddOn trials successfully met their primary and all key secondary endpoints, demonstrating statistically significant and clinically meaningful greater reductions in low-density lipoprotein cholesterol (LDL-C) for enlicitide compared to placebo (CORALreef HeFH) and compared to other oral non-statin therapies (CORALreef AddOn). There were no clinically meaningful differences in incidences of adverse events (AE) and serious adverse events (SAE) in either trial.
Results from the three Phase 3 trials in the CORALreef clinical development program will be presented at a future scientific congress.
Key takeaways from CORALreef HeFH and CORALreef AddOn studies:
• CORALreef HeFH: statistically significant and clinically meaningful reductions in LDL-C for enlicitide versus placebo in adults with heterozygous familial hypercholesterolemia (HeFH) who have a history of or are at risk for atherosclerotic cardiovascular disease (ASCVD) and are treated with a statin.
• CORALreef AddOn: statistically significant and clinically meaningful reductions in LDL-C for enlicitide versus ezetimibe, versus bempedoic acid and versus ezetimibe and bempedoic acid in adults with hyperlipidemia who have a history of or are at risk for ASCVD and are treated with a statin.
“We are thrilled to bring forward the first Phase 3 results from our clinical development program evaluating enlicitide, which, if approved, would be the first marketed oral PCSK9 inhibitor in the U.S.,” said Dr. Dean Y. Li, president, Merck Research Laboratories. “Enlicitide is a novel macrocyclic peptide that has the potential to deliver antibody-like efficacy and specificity for the validated PCSK9 mechanism in the form of a daily oral pill. We are working with urgency to make this oral therapy available to patients worldwide.”
“Atherosclerotic cardiovascular disease accounts for 85 percent of cardiovascular deaths. Despite available treatment options, cardiovascular-related deaths remain the leading cause of death worldwide and continue to rise,” said Dr. Christie M. Ballantyne, principal investigator of the CORALreef HeFH study and Professor of Medicine at Baylor College of Medicine. “LDL-C is a major modifiable risk driver for atherosclerosis and prioritization of LDL-C management should be a cornerstone of cardiovascular risk prevention. Early intervention and intensification of lipid treatment would allow more patients to achieve LDL-C goals.”
The efficacy and safety of enlicitide are being evaluated through the comprehensive CORALreef Phase 3 clinical development program, which aims to enroll approximately 17,000 patients across several trials, including two large ongoing trials, CORALreef Lipids and CORALreef Outcomes.
About CORALreef HeFH
CORALreef HeFH (NCT05952869) is a Phase 3, randomized, double-blind, placebo-controlled, multicenter study designed to evaluate the safety and efficacy of enlicitide compared to placebo in adults with HeFH who have a history of or were at risk for a major ASCVD event and are treated with a moderate or high intensity statin with or without other lipid-lowering therapies. The primary endpoints were mean percent change from baseline in LDL-C at week 24, number of participants with one or more adverse events (AEs), and number of participants who discontinued study drug due to an AE. Secondary endpoints included mean percent change from baseline in LDL-C at week 52, mean percent change from baseline in non-HDL-C, ApoB and percent change in Lp(a) at week 24.
About CORALreef AddOn
CORALreef AddOn (NCT06450366) is a Phase 3, randomized, double-blind, multicenter study designed to evaluate the efficacy and safety of enlicitide compared to ezetimibe, to bempedoic acid, and to ezetimibe and bempedoic acid, in patients with hypercholesterolemia who had a history of a major ASCVD event or were at risk for a major ASCVD event and are treated with a statin. The primary endpoint was the mean percent change from baseline in LDL-C at week 8. Secondary endpoints included mean percent change from baseline in non-HDL-C and ApoB.
About enlicitide and PCSK9
Enlicitide is an investigational, potentially first oral PCSK9 inhibitor designed to lower LDL-C via the same biological mechanism as currently approved monoclonal antibody injectable PCSK9 inhibitors but in a daily pill form. Enlicitide is a novel oral macrocyclic peptide that binds to PCSK9 and inhibits the interaction of PCSK9 with LDL receptors.
PCSK9 plays a key role in cholesterol homeostasis by regulating levels of the LDL receptor, which is responsible for the uptake of cholesterol into cells. Inhibition of PCSK9 with enlicitide prevents the interaction of PCSK9 with LDL receptors. This results in greater numbers of LDL receptors available on the cell surface to remove LDL cholesterol from the blood.
About hyperlipidemia
Hyperlipidemia is a disorder characterized by an excess of lipids or fats in the blood, affecting approximately 86 million adults (aged 20 and older) in the U.S. Despite adjusting diet or other lifestyle factors, some individuals may not reach recommended lipid levels and will require medication to treat and manage hyperlipidemia. Hyperlipidemia is a major risk driver for the development of ASCVD events, such as heart attacks and strokes, which account for 85 percent of cardiovascular deaths.