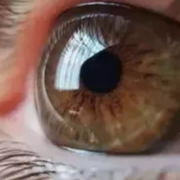
Retinitis pigmentosa patients may experience new depressive episodes, finds JAMA study
A new large-scale study published in the Journal of American Medical Association highlighted the increased risk of depressive disorder among patients diagnosed with retinitis pigmentosa (RP) that causes progressive vision loss. This population-based cohort study revealed that individuals with RP face a significantly higher likelihood of developing depressive symptoms when compared to the general population.
The research analyzed data from the Health Insurance Review and Assessment Service in Korea between 2008 and 2022 and involved 10,879 individuals newly diagnosed with RP from 2011 to 2021. The objective of this research was to examine the link between RP and depressive disorder, particularly in the context of different age groups and sexes.
The study found that the 10-year cumulative incidence rate of depressive disorder in RP patients, which stood at 17.67%. The study also reported that older patients and women with RP were at even higher risk of developing depression. Also, women had a 46% greater risk of depression when compared to men. Additionally, the individuals aged 40 and older were nearly twice as likely to develop depression than the younger RP patients.
The patients were categorized into 3 groups based on their age at diagnosis as under 20 years old, between 20 and 39 years old and 40 years or older. The study then calculated age- and sex-adjusted standardized incidence ratios (SIRs) of depressive disorder by revealing that RP patients had an overall SIR of 1.19 which indicated a 19% higher risk of depression than the general population. This increased risk was present in both men and women, though it was slightly higher in female patients. Age-based analysis showed a trend where the risk of depression was 50% higher than in the general population. The patients aged 60 and older also showed an elevated SIR of 1.25.
The results of this study indicated that individuals with RP who were older adults and women are particularly vulnerable to depression, likely due to the emotional and psychological toll of living with progressive vision loss. These findings highlight the critical need for emotional and mental health support for RP patients, alongside the management of their physical symptoms. Overall, the study emphasized the importance of early detection and treatment of depressive symptoms in RP patients to improve their overall quality of life.
Reference:
Kim, H. R., Lee, N. K., Suh, Y., Lee, C. S., Byeon, S. H., Kim, S. S., Lee, S. W., & Kim, Y. J. (2024). Incidence and Risk of Depressive Disorder in Patients With Retinitis Pigmentosa. In JAMA Ophthalmology. American Medical Association (AMA). https://doi.org/10.1001/jamaophthalmol.2024.3641
Powered by WPeMatico