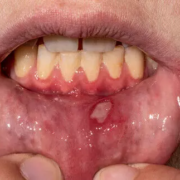
Oral roflumilast safe and effective therapy for patients with recurrent aphthous stomatitis: Study
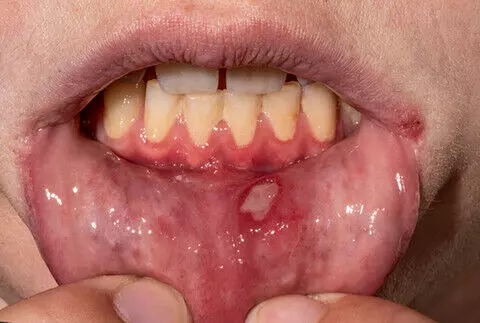
A study published in the International Journal of Dermatology suggests that oral roflumilast is a safe and effective therapy for patients with recurrent aphthous stomatitis.
Severe recurrent aphthous stomatitis (RAS) represents a therapeutic challenge because of its impact on the patient’s quality of life. Additionally, no approved systemic therapies are available. Roflumilast, a phosphodiesterase-4 inhibitor, has shown promise in other inflammatory dermatological conditions. This study aimed to assess the characteristics, effectiveness, and safety of roflumilast in treating RAS in routine clinical practice. This is a single cohort ambispective observational study conducted in five Spanish centers. Twenty-two patients with RAS treated with roflumilast participated. Data collection included demographic, clinical, and outcome variables. Statistical analysis compared the outcomes of 12 weeks of roflumilast treatment with a similar prior period without treatment. Results: During treatment with roflumilast, a significant reduction in flare-ups (88%) and oral ulcers (94%) was observed compared to the untreated period. A reduction in pain (66%) and ulcer duration (63%) was observed. Adverse effects (AEs) occurred in 13 patients, predominantly headache and gastrointestinal disturbances. Most of these were self-limiting or manageable with dose adjustment. Treatment was withdrawn in three cases, mainly because of AEs. This study suggests that roflumilast may effectively treat RAS by reducing the number of flare-ups and ulcers, their duration, and the symptomatology produced by the ulcers. In addition, roflumilast has a good safety profile, is well tolerated at low doses, and does not require close monitoring. These characteristics and its favorable economic profile make roflumilast a promising therapeutic option in this pathology.
Reference:
Peñuelas Leal, R., Bagan, L., Grau Echevarría, A., Peñuelas Ruiz, J.A., Zaragoza Ninet, V., Sánchez Carazo, J.L., Pérez Pastor, G., Labrandero Hoyos, C., Finello, M., Martínez Fernández, S., Blaya Imbernon, D., González García, Á., Pérez Zafrilla, E., Martí Cabrera, M. and Bagan, J. (2024), Treatment of recurrent aphthous stomatitis with oral roflumilast, a multicenter observational study. Int J Dermatol. https://doi.org/10.1111/ijd.17478
Keywords:
Oral, roflumilast, safe, effective, therapy, patient, recurrent, aphthous, stomatitis, study, International Journal of Dermatology, Peñuelas Leal, R., Bagan, L., Grau Echevarría, A., Peñuelas Ruiz, J.A., Zaragoza Ninet, V., Sánchez Carazo, J.L., Pérez Pastor, G., Labrandero Hoyos, C., Finello, M., Martínez Fernández, S., Blaya Imbernon, D., González García, Á., Pérez Zafrilla, E., Martí Cabrera
Powered by WPeMatico