Arginine dentifrices significantly reduce childhood caries, clinical trial finds
Powered by WPeMatico
Powered by WPeMatico
Powered by WPeMatico
Powered by WPeMatico
Powered by WPeMatico
Powered by WPeMatico
Powered by WPeMatico
Powered by WPeMatico

India: A large multicentre clinical trial conducted in India has found that three commonly prescribed dual-drug regimens for high blood pressure are equally effective and well-tolerated in South Asian adults. The findings, published in Nature Medicine, provide important evidence to guide hypertension management in the region, where cardiovascular disease remains a major cause of illness and death.
Led by Dorairaj Prabhakaran and colleagues from the Centre for Chronic Disease Control in New Delhi, the study aimed to address a significant evidence gap in optimising combination therapy for South Asian patients. While dual therapy is often recommended when single medications fail to achieve adequate blood pressure control, there has been little comparative data on which combinations work best in this population.
The trial enrolled 1,981 Indian adults aged 30 to 79 years, with a mean age of 52 years. Participants had either a sitting systolic blood pressure (SBP) of 150–179 mmHg while untreated, or an SBP of 140–159 mmHg while already on a single medication. They were randomly assigned, in equal proportions, to receive one of three single-pill combinations: amlodipine–perindopril, perindopril–indapamide, or amlodipine–indapamide. The primary goal was to measure changes in 24-hour ambulatory SBP after six months of treatment.
The study led to the following findings:
The trial, known as the TOPSPIN trial, is one of the largest head-to-head comparisons of dual therapy in Indian patients. According to the authors, the results suggest that any of these combinations—amlodipine–perindopril, perindopril–indapamide, or amlodipine–indapamide—can be considered equally valid first choices for initiating dual therapy in South Asian adults with hypertension.
Given that elevated blood pressure is a leading risk factor for heart attacks, strokes, and kidney disease, the researchers believe the findings will help clinicians tailor treatment strategies without being restricted to one specific combination. The comparable efficacy and safety profiles of these regimens may also allow flexibility based on patient preferences, cost, and availability.
By providing robust, region-specific evidence, the study addresses a critical gap in hypertension management for South Asians, both in India and across the global diaspora, potentially improving blood pressure control and reducing cardiovascular disease burden.
Reference:
Prabhakaran, D., Roy, A., Chandrasekaran, A. M., Kondal, D., Mukherjee, S., Kiru, G., Singh, K., Salwa, H., Sobitharaj, E. C., Lobo, A. S., Mahajan, G., Mohan, B., Khanna, A., Malviya, A., Patil, S. G., Abichandani, V. K., Singh, B., Gupta, B. K., Yellapantula, B., . . . Poulter, N. R. (2025). Comparison of dual therapies for hypertension treatment in India: A randomized clinical trial. Nature Medicine, 1-7. https://doi.org/10.1038/s41591-025-03854-w
Powered by WPeMatico
Powered by WPeMatico
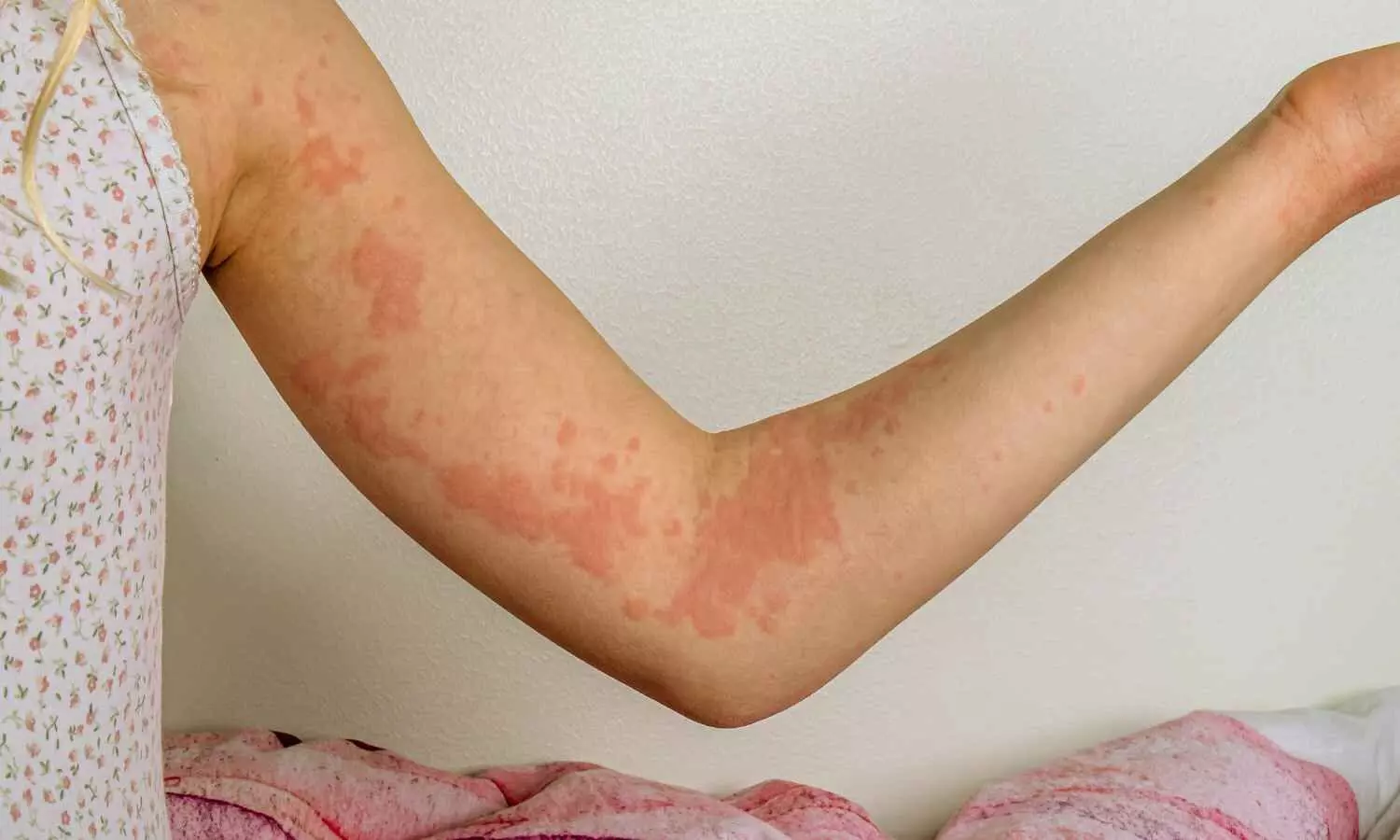
A new study published in the Journal of American Medical Association found that Rilzabrutinib decreased itching and hives while preserving a positive risk-benefit profile, indicating that it might be a useful therapy for individuals with moderate to severe chronic spontaneous urticaria (CSU) that is resistant to antihistamines.
The primary cause of CSU is the activation of cutaneous mast cells via a variety of pathways. B cells and mast cells contain the protein bruton tyrosine kinase (BTK), which is essential for several immune-mediated disease processes. Thus, this research evaluated the effectiveness and risk profile of rilzabrutinib, a covalent, oral, reversible, next-generation BTK inhibitor, in the treatment of patients with CSU.
The Rilzabrutinib Efficacy and Safety in CSU (RILECSU) randomized clinical trial was a 52-week phase 2 research that included a 40-week open-label extension after a 12-week double-blind, dose-ranging, placebo-controlled period. From November 24, 2021, until April 23, 2024, the trial was held.
12 countries across Asia, Europe, North America, and South America, 51 centers recruited and randomly assigned participants. Adults with moderate to severe CSU (weekly Urticaria Activity Score [UAS7] of 16 or more; weekly Itch Severity Score [ISS7] of 8 or higher) who were not well managed with H1-antihistamine medication were enrolled in the experiment. This ranged in age from 18 to 80 and the patients were randomized 1:1:1:1 to 400 mg of rilzabrutinib, 400 mg once a day in the evening, 800 mg twice daily, 1200 mg three times daily, or a matched placebo.
160 omalizumab-naive and omalizumab-incomplete responders (mean [SD] age, 44.1 [13.4] years; 112 [70.0%] females) were randomly assigned. Only the 143 individuals who had never used omalizumab were part of the primary analysis population. ISS7 (least squares [LS] mean, −9.21 vs −5.77; difference, −3.44 [95% CI, −6.25 to −0.62]; P =.02) and UAS7 (LS mean, −16.89 vs −10.14; difference, −6.75 [95% CI, −12.23 to −1.26) showed significant decreases at week 12 when rilzabrutinib, 1200 mg/d, was compared to placebo from baseline.
Improvements were also seen in the weekly Angioedema Activity Score (AAS7) and weekly Hives Severity Score (HSS7). As early as week 1, ISS7, UAS7, HSS7, and AAS7 showed improvements. At week 12, CSU-related biomarkers, such as immunoglobulin (Ig)-G antithyroid peroxidase, soluble Mas-related G protein–coupled receptor X2, IgG anti-Fc-ε receptor 1, and interleukin-31, were lower than placebo.
Overall, along with an acceptable adverse event profile, the RILECSU randomized clinical trial findings showed that rilzabrutinib, 1200 mg/d, was effective and had a quick start of action over a 12-week period.
Reference:
Giménez-Arnau, A., Ferrucci, S., Ben-Shoshan, M., Mikol, V., Lucats, L., Sun, I., Mannent, L., & Gereige, J. (2025). Rilzabrutinib in antihistamine-refractory chronic spontaneous urticaria: The RILECSU phase 2 randomized clinical trial: The RILECSU phase 2 randomized clinical trial. JAMA Dermatology (Chicago, Ill.), 161(7), 679–687. https://doi.org/10.1001/jamadermatol.2025.0733
Powered by WPeMatico
