New Delhi: The National Consumer Disputes Redressal Commission (NCDRC) recently exonerated a UP-based hospital and its two doctors from allegations of medical negligence while administering anaesthesia to a patient before conducting a laparoscopic cholecystectomy.
Even though, the State Commission had directed them to pay a hefty compensation to the patient who lost sensation in her right leg and faced permanent disability, the Apex Consumer Court exonerated them relying on the MRI report and the expert opinion given by SGPGI.
The history of the case goes back to 2014 when the patient suffered sudden abdomen pain and consulted a doctor, who advised for ultrasound of the abdomen and pelvis bone. The report showed “Cholecystitis with echogenic gall bladder sludge. Small left renal concretion”.
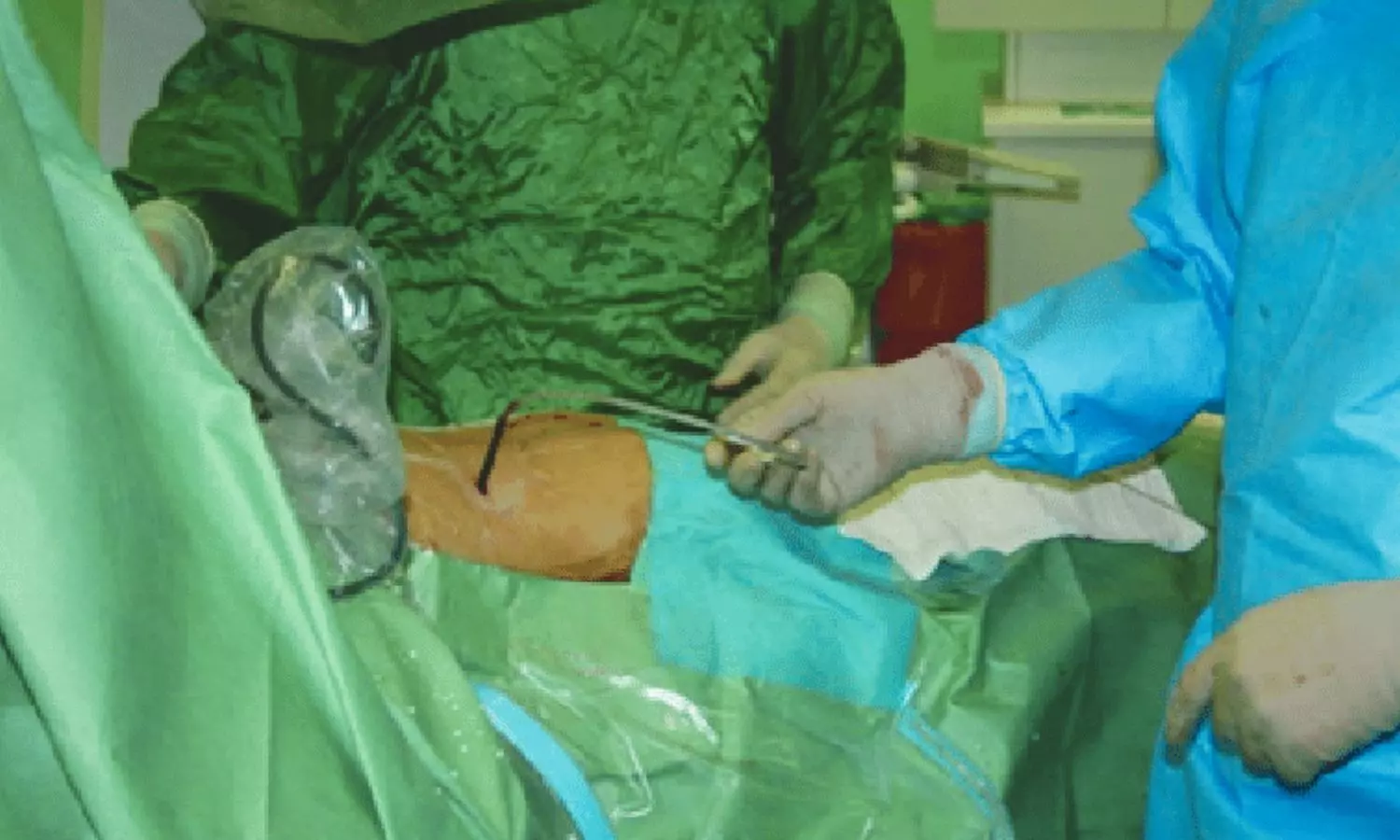
When the patient showed the ultrasound report to the treating doctor, he advised certain tests and medication. Thereafter, the patient was admitted to Opal Hospital and the treating doctor conducted the laparoscopic cholecystectomy. It was alleged that after being administered spinal anaesthesia, the patient felt current like shock sensation in her right leg. Immediately, one of the treating doctors took out the needle and inserted it again in the patient’s spine. Against, she felt a current-like sensation. After that, the staff of the hospital held the patient and the doctor inserted the needle for a third time. After administering the anaesthetic medicine, Dr. Rai conducted the surgery.
However, after regaining consciousness, when the patient tried to move her right leg, she was unable to do so although other body parts were working normally. It was further alleged that despite informing the doctors, they did not pay attention to it. After removing the catheter, the patient realised that there was no sensation in her right leg, which was virtually paralyzed. Consequently, an Orthopaedic Surgeon was called.
Noting that there was no report by the Anaesthetist, the patient’s husband called for the opinion of Dr. Rakesh Singh, a neurosurgeon and Dr. D K Singh, an Anaesthetist. After examining the patient, Dr. Rakesh Singh observed as “(a) Patient not able to walk on 17 Th unable to put right foot properly on ground. (b) O/E not able to dorsiflex right foot. (c) Slight movement on fingers present, sensation on foot absent. (d) Case discussed with orthopaedic surgeon and treatment given as per advice.”
The patient was advised not to walk without any accompanying person to avoid falls. Meanwhile, the treating doctor advised an MRI of the Lumbosacral Spine and the report mentioned, “MRI features of focal increased, Intramedullary signal intensity in the conus meddullar is on T2 weighted images at D12-L1 level as described above. Early Lumbar Spondylosis with mild anterior subluxation of L4 over L5 vertebral body with annular bulge L4/5 I.V. Disc with attendant B/L facet joint arthrosis with hypertrophied ligamentum flavum leading to thecal sac indentation with mild B/l neural foramina encroachment.”
Referring to the MRI report, the complainant argued that the damage to the spinal cord was done while injecting anaesthetic drugs and the damage caused was so severe that could not be reverted back to normal functioning of the right leg.
Despite undergoing treatment for several days, the lack of sensation in the right leg continued and the patient was discharged in that condition. The treating Orthopaedic Surgeon observed “Full right foot drop post spinal. Pain in calf. Tender, mild.”
After this, the patient got herself examined at several other hospitals including IMS, BHU, Max Hospital Delhi, Primus Super Specialty Hospital Delhi, etc. and allegedly she was undergoing treatment even during proceedings before the Consumer Court.
Therefore, alleging that the Anaesthetist was grossly negligent and deficient in administering the spinal anaesthesia to the patient resulting in permanent disability, the plaintiff filed a complaint and prayed for a hefty compensation.
On the other hand, the hospital and doctors denied the allegations and insisted that there was no medical negligence on their part. Referring to the MRI report, they argued that from the report no one can say that the lesion is because of spinal anaesthesia and cannot be reversed back. It was submitted that there was a good response in the patient’s right leg and the patient could put heal on the ground but there was no response in the toe.
After hearing the parties, the State Commission found that a common rule in the field of anaesthesia was that if you prick the spinal first time and the patient suffers an electric shock, then it must be aborted. Meanwhile, admittedly, the anaesthetist pricked twice and the expert opinion of SPPGI did not mention as to how many times the anaesthetic needle can be pricked if the first prick fails.
The State Commission had also observed that there was no separate consent for anaesthesia, and the consent form was not in prescribed proforma. Due to defective spinal anaesthesia and lack of knowledge regarding anaesthesia resulted in the pity situation of the patient, which resulted in 60% disability in the patient. Foot drop is a neurological disorder, which occurs following natural childbirth and spinal anaesthesia due to direct needle trauma or local anaesthetic toxicity, the State Commission had noted in its order dated 09.03.2023. It had directed the hospital and the doctors to pay Rs 15 lacs as compensation, Rs 10 as doctor’s fees and other medical expenses, Rs 1 lac for extra nourishment, and Rs 60 lacs for permanent burning, sensation, pain, suffering, disfigurement of right leg, physical and mental agony and litigation cost with interest @10% per annum from 01.01.2019 till the date of payment to the complainant.
This order was challenged by the hospital and the doctors before the Apex Consumer Court, which noted that the patient came to the hospital for laparoscopic cholecystectomy and the patient’s husband signed the Consent Form which was a composite form for operation and anaesthesia.
“The complainant did not raise any issue in respect of the consent that ‘informed consent’ was not obtained. But State Commission has recorded adverse findings in this respect without there being any issue between the parties in respect,” the NCDRC bench observed.
Referring to Supreme Court orders in the case of Samira Kohli v. Dr. Prabha Manchanda, M.A. Biviji v. Sunita and V. Kishan Rao v. Nikhil Super Speciality Hospital, the top consumer court observed,
“Although, expert opinion is not necessary in every case but expert opinion requires its evaluation cautiously in corroboration of other evidence on record and standard medical literature.”
It was noted by NCDRC that the State Commission ignored the Expert Opinion of SGPGI because it did not mention how many times the anaestheic needle can be pricked if the first prick fails and further did not say about puncturing the spinal nerve during the spinal anaesthesia. The expert did not consider the current-like sensation felt by the patient, it further noted.
Further, the Commission noted that there was no allegation that there was any negligence committed during the surgery of the gall bladder or post-surgery and there was any complication in this regard. The allegations were that the anaesthesia had been administered in a supine condition, the needle was inserted three times, while in first attempt itself and in all attempts, the patient experienced shock-like sensation. The NCDRC bench further noted that the hospital and doctors denied these allegations.
“The spinal anaesthesia cannot be administered in supine condition which is impossibility. Thus the allegation of the complaint in this respect is not liable to be believed. Similarly, the allegation that the patient experienced shock sensation in her right leg in sprinkling the needle has also been denied. The opposite parties stated that the patient was asked to set relaxed in blow down position with head down and in second attempt the anaesthesia has been administered without any help of the other medical staff and there was no complaint about shock sensation in right leg by the patient. OP-3 is an experienced qualified anaesthetist. There is no reason to disbelieve the statement of the OPs in this respect. Similarly presence of OP-3 in operation theatre on 15.02.2014, administering anaesthesia by her are admitted as such there is no reason to disbelieve the records of presurgery assessment of the patient and monitoring the patient during surgery prepared by the OP-3,” the Commission observed at this outset.
While examining the question of whether the complication had been caused due to giving anaesthesia in any wrong or negligent manner, the Commission took note of the MRI report dated 18.02.2014 on record.
“Overall, the MRI findings suggest that the patient has early-stage degenerative changes in her lumbar spine. The mild subluxation, annular bulge, facet joint arthrosis, and ligamentum flavum hypertrophy are all contributing to potential nerve compression. MRI features of focal increased intramedullary signal intensity in the conus medullaris on T2 weighted images at D12-L1 level are often indicative of a pathological process within the spinal cord. Given these MRI findings, nonspecific myelitis is a highly probable diagnosis. This condition involves inflammation of the spinal cord without a clearly identifiable cause. The increased signal intensity on T2-weighted imaging is often a hallmark of inflammation within the spinal cord. The expert of SPPGI has not accepted the allegation of the complainant that these complications developed due to wrong manner of needle prinks. In the absence of any contradictory expert opinion or any standard medical literature, it is not possible to find that OP-3 has committed any medical negligence in administering anaesthesia,” noted the Commission.
“From MRI report no one can say that lesion is because of spinal anaesthesia and cannot be reversed back. There was good response in right leg. The patient could put heel on ground but no response in toe. The patient was feeling better and asked for discharge and she was discharged on 19.02.2014 in morning,” it further noted and accordingly set aside the order of the State Commission.
To view the order, click on the link below:
https://medicaldialogues.in/pdf_upload/ncdrc-exonerates-257531.pdf
Also Read: Surgeon Cannot be held responsible for gangrene: Consumer Court relief