Compared to surgical removal of mandibular third molars, Coronectomy significantly impacted postoperative oral health related QoL: Study
Compared to surgical removal of mandibular third molars, Coronectomy significantly impacted postoperative oral health-related QoL suggests a study published in the Journal of Oral and Maxillofacial Surgery.
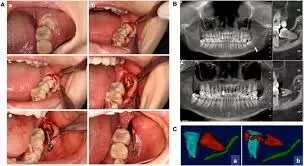
Coronectomy is an alternative procedure for removing mandibular third molars near the inferior alveolar nerve. Limited research exists on the effect of coronectomy on the postoperative quality of life (QoL). This study compared postoperative QoL after coronectomy and complete surgical removal of mandibular third molars during the first postoperative week. This prospective cross-over study was conducted in the Oral and Maxillofacial Department of Amstelland Hospital, Amstelveen, The Netherlands. The study sample consisted of patients with indications for removal of both mandibular third molars, with one at increased risk of nerve injury undergoing coronectomy, while the other molar was extracted. Exclusion criteria were ibuprofen allergy, anticoagulant therapy, systemic disease, local pathology, or failure to complete the Oral Health Impact Profile-14 (OHIP-14) questionnaire. Results: The sample included 55 patients (18 male and 37 female) with a mean age of 24.6 ± 4.7 years. Mean OHIP-14 scores during the first 6 postoperative days were significantly higher after coronectomy compared to after surgical removal (day 1: 24.93 ± 9.82 vs 22.7 ± 9.5; day 6: 11.27 ± 10.36 vs 8.49 ± 10.93) (P < .05). Pain was significantly higher on the second to sixth postoperative days after coronectomy (day 2: 6.02 ± 1.92 vs 5.78 ± 1.73; day 6: 4.11 ± 2.49 vs 3.09 ± 2.41) (P < .05). Patients used more analgesics after coronectomy (day 2: 4.09 ± 2.53 vs 3.27 ± 1.9; day 6: 2.76 ± 2.62 vs 2.13 ± 2.49) (P < .05). They found no differences in outcomes for sex or molar impaction (P > .05). Coronectomy significantly impacted postoperative oral health–related QoL compared to complete surgical removal of mandibular third molars.
Reference:
Postoperative Outcome After Coronectomy Versus Surgical Removal of Impacted Mandibular Third Molars – Clinical and Oral Health-Related Quality of Life Follow-Up
Simons, Rashida N. et al. Journal of Oral and Maxillofacial Surgery, Volume 82, Issue 9, 1109 – 1120
Keywords:
Compared, surgical, removal, mandibular, third molars, Coronectomy, significantly, impacted, postoperative, oral health related QoL, Study, Journal of Oral and Maxillofacial Surgery
Powered by WPeMatico