Single-Dose Clesrovimab Safely Lowers RSV Infections and Hospitalizations in Infants: NEJM
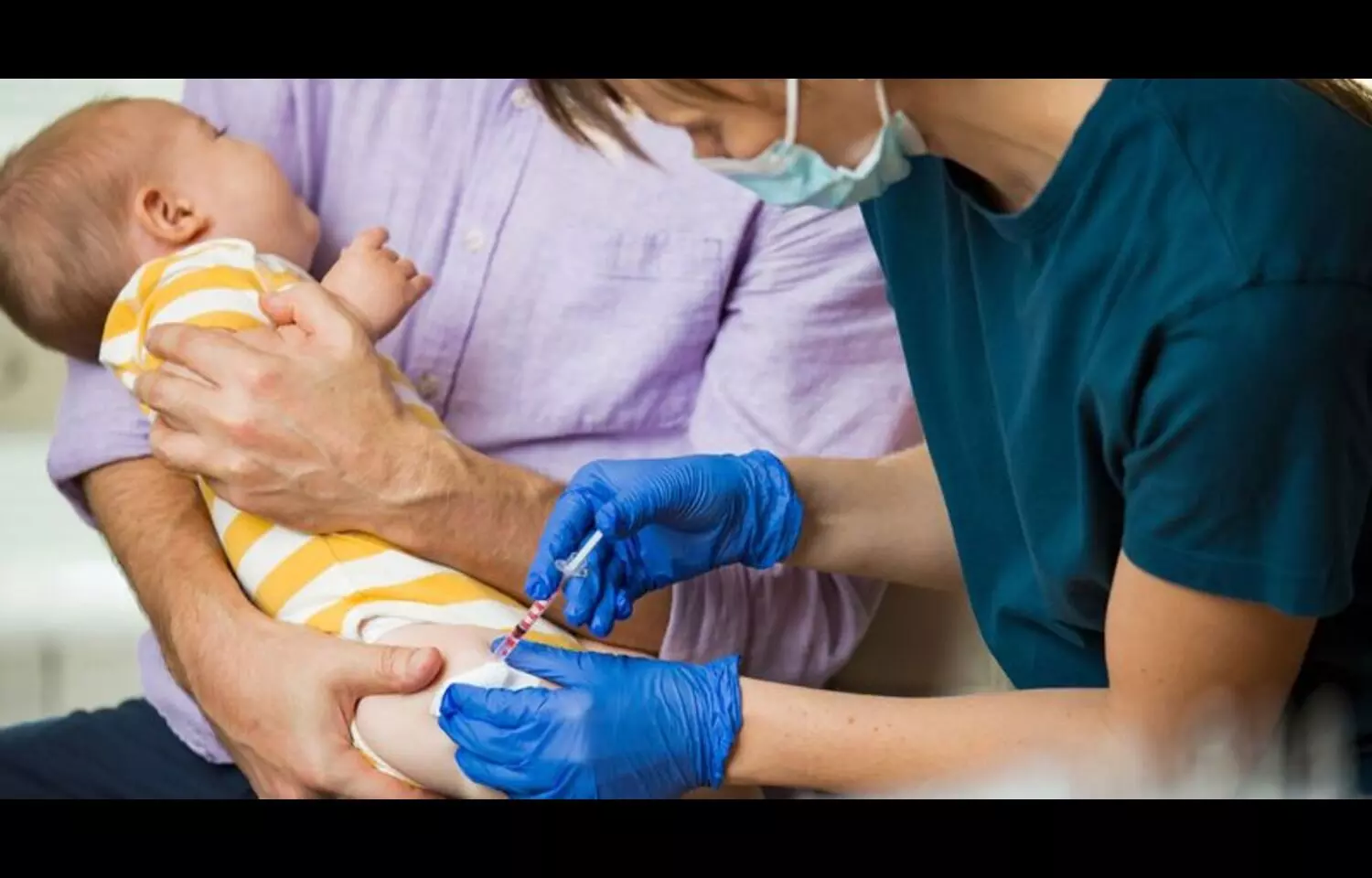
Researchers have found in a new clinical trial that a single dose of clesrovimab, a long-acting investigational monoclonal antibody targeting site IV of the respiratory syncytial virus (RSV) fusion protein, significantly reduced the risk of RSV-associated illness in healthy infants. The study, published in The New England Journal of Medicine, evaluated the safety and efficacy of clesrovimab in both preterm and full-term infants during their first RSV season, a period marked by heightened vulnerability to severe lower respiratory tract infections. Infants were randomly assigned to receive either one intramuscular injection of clesrovimab or placebo, and outcomes were monitored for 150 days following administration. Findings showed that clesrovimab markedly lowered the incidence of medically attended lower respiratory tract infections due to RSV compared with placebo, and also substantially reduced RSV-related hospitalizations, underscoring its potential as a preventive strategy for this common and sometimes life-threatening infection. Importantly, the safety profile of clesrovimab was comparable to placebo, with no signal of increased adverse events, further supporting its potential suitability for widespread use. RSV is a leading cause of infant hospitalization worldwide, and while supportive care remains the mainstay of treatment, there is a recognized need for effective preventive measures beyond maternal vaccination and existing monoclonal antibodies. The results of this large, randomized, placebo-controlled trial suggest that clesrovimab could fill an important gap in infant RSV prevention strategies by providing durable protection with a single dose, potentially easing the burden on healthcare systems and reducing parental anxiety during RSV season. The authors noted that longer follow-up and real-world studies will be essential to confirm durability of protection, cost-effectiveness, and feasibility of integration into national immunization programs, but the current evidence positions clesrovimab as a promising candidate for future inclusion in RSV prevention protocols for infants.
Keywords: Clesrovimab, RSV, monoclonal antibody, infants, prevention, respiratory infection, hospitalization, immunization
Reference: Zar HJ, Simões EAF, Madhi SA, Ramilo O, Senders SD, Shepard JS, Laoprasopwattana K, et al; CLEVER (MK-1654-004) Study Group. Clesrovimab for Prevention of RSV Disease in Healthy Infants. N Engl J Med. 2025; DOI: 10.1056/NEJMoa2502984.
Powered by WPeMatico