Study sheds light on hurdles faced in transforming NHS health care with AI
Powered by WPeMatico
Powered by WPeMatico
Powered by WPeMatico
Powered by WPeMatico
Powered by WPeMatico
Powered by WPeMatico

India: A recent study published in Diabetologia has shed light on a unique presentation of type 2 diabetes among young adults in Assam, India-one that defies the conventional link between diabetes and obesity.
Conducted by Dr. Anupam Dutta and colleagues from the Department of Medicine, Assam Medical College and Hospital, Dibrugarh, the PHENOEINDY-2 study highlights a concerning prevalence of non-autoimmune diabetes among undernourished individuals, with beta cell dysfunction emerging as the primary driver.
The study involved 240 GADA-negative young adults (diagnosed with type 2 diabetes before the age of 40) and 252 non-diabetic individuals from similar socio-economic backgrounds as controls. Notably, both groups shared a median BMI of 23 kg/m². Many participants came from economically disadvantaged tea garden communities, a setting that offered critical insights into the interplay between poverty, malnutrition, and metabolic disorders.
The key findings of the study were as follows:
Further classification revealed that around two-thirds of the participants with diabetes belonged to the “severely insulin-deficient diabetes” (SIDD) subgroup based on the Swedish classification system. These findings point toward beta cell deficiency, rather than insulin resistance, as the primary abnormality in this population.
The authors suggest that multigenerational undernutrition may play a crucial role in the development of diabetes in this setting. The study underscores the need to broaden the understanding of diabetes phenotypes in low- and middle-income countries and tailor interventions accordingly.
“The PHENOEINDY-2 study calls attention to the heterogeneity of young-onset type 2 diabetes in India. In regions like Assam, where undernutrition remains a persistent issue, the conventional obesity-linked model of diabetes may not apply. Instead, chronic nutritional deprivation and associated beta-cell impairment appear to be at the heart of the disease’s development,” the authors concluded.
Reference:
Dutta, A., Dutta, P.K., Baruah, S.M. et al. Non-autoimmune diabetes in young people from Assam, India: the PHENOEINDY-2 study. Diabetologia (2025). https://doi.org/10.1007/s00125-025-06500-9
Powered by WPeMatico

New Delhi: The Delhi High Court has directed the Central Drugs Standard Control Organisation (CDSCO) not to enforce its order initiating criminal prosecution against IndiaMART Intermesh Limited over alleged listings of illegal drugs on its online platform.
Justice Saurabh Banerjee, while posting the matter before the roster bench for hearing next week, ordered that CDSCO’s directive of September 1, 2025, “shall remain in abeyance until September 17, 2025.”
According to a recent media report in the Bar and Bench, appearing for IndiaMART, Senior Advocate Darpan Wadhwa, assisted by Advocate Anirudh Bakru and a team from Bahuguna Law Associates, argued that the provisions invoked—Sections 18 and 27 of the Drugs and Cosmetics Act, 1940 — govern manufacturers, sellers, and distributors of medicines and cannot be applied to an online marketplace.
IndiaMART further maintained that it functions solely as an intermediary under the Information Technology Act, does not participate in transactions between buyers and sellers, and is protected by the safe harbour provisions of Section 79 of the IT Act.
The company likened its role to a digital directory or “yellow pages,” stating that it only hosts product listings created by third-party vendors, and has no involvement in the manufacturing or sale of drugs.
The CDSCO’s counsel, while reserving the regulator’s rights, informed the Court that the September 1 order would not be acted upon until the case is heard.
The dispute traces back to multiple notices and letters issued by CDSCO between June 2024 and July 2025 — dated June 13, 2024; December 24, 2024; May 9, 2024; May 1, 2025; July 8, 2025; July 9, 2025; and July 18, 2025 — warning IndiaMART of potential prosecution and directing it to take corrective action.
IndiaMART had first approached the High Court in June 2024, challenging these notices. On July 22, 2025, Justice Sachin Datta recorded the submissions of government counsel that the company’s responses were under consideration and that a reasoned order would be passed after granting a hearing. At that stage, the Court had protected the company and its directors from immediate coercive action, observing that apprehension of prosecution was unfounded.
Following the CDSCO’s recent decision to initiate prosecution, IndiaMART once again sought the Court’s intervention, reiterating its stand that it is only an intermediary platform and not a trader in pharmaceutical products.
The company reiterated that it functions solely as an intermediary, similar to a yellow pages directory. It contended that its role is limited to listing products, with no involvement in their manufacture or sale, reports Bar and Bench.
Powered by WPeMatico

New Delhi: The Subject Expert Committee (SEC) under the Central Drugs Standard Control Organization (CDSCO) has recommended approval of an updated package insert for Rybelsus tablets (3 mg, 7 mg, and 14 mg), manufactured by Novo Nordisk (India) Private Limited.
The decision came during the SEC meeting held on August 20, 2025, at CDSCO headquarters in New Delhi. Novo Nordisk presented its proposal to update the package insert (Version February 2025) in line with the European Medicines Agency (EMA) approval.
The firm presented the proposal for update in Package Insert (Version February 2025) for the changes in the Sections of Special Warnings and Precautions for use, Drug Interactions, Use in special populations and Undesirable effects of the drug product Rybelsus 3 mg, 7mg and 14 mg tablets in line with EMA approval.
After detailed deliberation, the committee recommended, “for approval of updated package insert Version February 2025 of the said drug product for the proposed changes.”
Rybelsus (oral semaglutide) is a glucagon-like peptide-1 (GLP-1) receptor agonist indicated for the treatment of type 2 diabetes mellitus. It lowers blood glucose by enhancing insulin secretion, reducing glucagon, and delaying gastric emptying. Unlike most GLP-1 receptor agonists, which require subcutaneous injection, Rybelsus is taken orally once daily, providing a non-injectable option for patients.
Novo Nordisk, the innovator of semaglutide, markets both the injectable (Ozempic) and oral (Rybelsus) formulations globally. The updated package insert ensures that healthcare professionals in India receive the latest approved safety, interaction, and usage information aligned with EMA standards, helping clinicians optimize diabetes management.
Powered by WPeMatico
USA: A new study published in JAMA Network Open by researchers from the Mayo Clinic Vaccine Research Group has found concerning immunity gaps among vaccinated children in southern India, raising questions about the effectiveness of measles vaccination strategies in achieving elimination goals.
Powered by WPeMatico
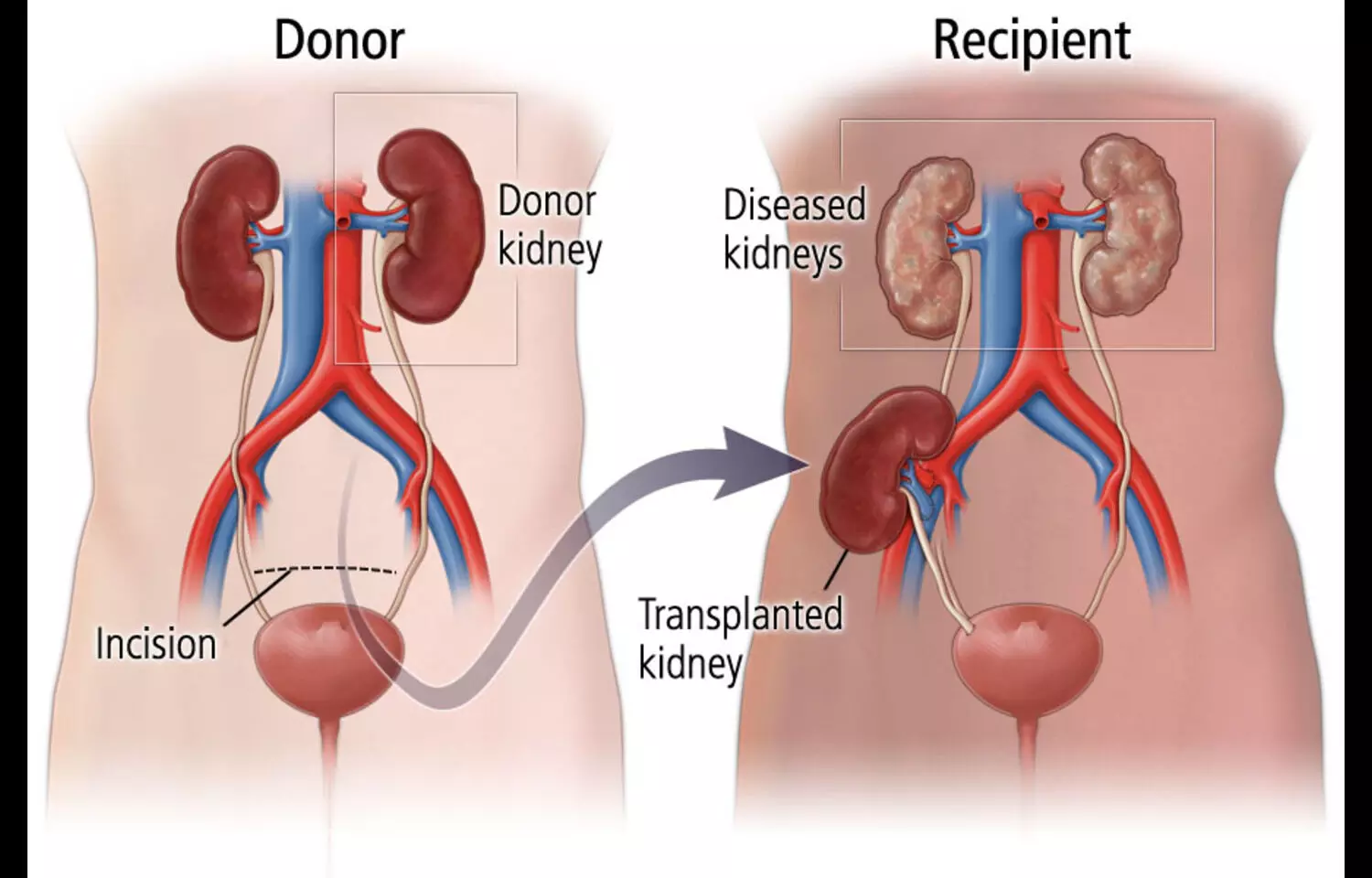
Researchers have found in a new study steroidal mineralocorticoid receptor antagonists (MRAs) show no superior efficacy over placebo in kidney transplant recipients and are linked to a fourfold increased risk of hyperkalemia, even with preserved kidney function.
In patients with chronic kidney disease, mineralocorticoid receptor antagonists (MRAs) exert a reno-protective effect through its anti-inflammatory and antifibrotic effects. Less is known about the efficacy of MRAs in kidney transplant (KT) recipients.
This meta-analysis aims to systematically assess the efficacy of MRAs in KT recipients. PubMed, Embase and Cochrane databases were searched for randomized controlled trials (RCTs) that compared MRAs to placebo in KT recipients and reported the outcomes of (1) glomerular filtration rate (GFR); (2) serum creatinine; (3) systolic (SBP) and diastolic blood pressure (DBP); (4) hyperkalemia; and (5) interstitial fibrosis and tubular atrophy (IFTA) scores.
Heterogeneity was examined with I2 statistics. A random-effects model was used for outcomes with high heterogeneity.
Results: They included 5 RCTs with 293 patients, of whom 142 (48.5%) underwent treatment with a steroidal MRA. Mean follow-up ranged from 5 days to 36 months. There was no significant difference in GFR (MD 9.04 mL/min/1.73 m2; 95% CI − 2.76–20.85; p = 0.13) and serum creatinine between placebo and MRA groups (MD − 0.21 mg/dL; 95% CI − 0.62–0.20; p = 0.32). SBP (MD 0.69 mmHg; 95% CI − 0.69–2.08; p = 0.33), DBP (MD 0.45 mmHg; 95% CI − 0.69–1.59; p = 0.44) and IFTA scores exhibited no differences between groups (mild IFTA RR 1.21; 95% 0.83–1.74; p = 0.32) (moderate IFTA RR 0.82; 95% CI 0.45–1.50; P = 0.51) (severe IFTA RR 0.64; 95% CI 0.24–1.76; p = 0.39). MRAs were associated with a 4-fold increase in the risk of hyperkalemia compared with placebo (RR 4.06; 95% CI 1.46–11.28; p = 0.007). Steroidal MRAs have no superior efficacy compared with placebo in KT recipients and are associated with a 4-fold increase in the risk of hyperkalemia despite preserved kidney function.
Reference:
BMC Nephrology. (2025). Steroidal mineralocorticoid receptor antagonists ineffective and risky in kidney transplant recipients: Study. BMC Nephrology. https://bmcnephrol.biomedcentral.com/articles/10.1186/s12882-025-043XX-X [API]
Powered by WPeMatico
