Canada urged to back health-care developed solutions for innovation leadership
Powered by WPeMatico
Powered by WPeMatico
Powered by WPeMatico
Powered by WPeMatico
Powered by WPeMatico
Powered by WPeMatico

Ivermectin administered to the whole population significantly reduces malaria transmission, offering new hope in the fight against the disease. The BOHEMIA trial, the largest study on ivermectin for malaria to date, showed a 26% reduction in new malaria infection on top of existing bed nets, providing strong evidence of ivermectin’s potential as a complementary tool in malaria control. The results of this project, coordinated by the Barcelona Institute for Global Health (ISGlobal) -an institution supported by the “la Caixa” Foundation- in collaboration with the Manhiça Health Research Centre (CISM) and the KEMRI-Wellcome Trust Research Programme, have been published in The New England Journal of Medicine.
Malaria remains a global health challenge, with 263 million cases and 597,000 deaths reported in 2023. Current vector control methods, such as long-lasting insecticidal nets (LLINs) and indoor residual spraying (IRS), have become less effective due to insecticide resistance and behavioural adaptations in mosquitoes to bite outdoors and during dusk or dawn, when people are not protected by these measures. This underscores the urgent need for innovative solutions to combat malaria.
Ivermectin, a drug traditionally used to treat neglected tropical diseases like onchocerciasis, which causes river blindness, and lymphatic filariasis, which causes elephantiasis, has been shown to reduce malaria transmission by killing the mosquitoes that feed on treated individuals. Given the rising resistance to conventional insecticides, ivermectin could offer an effective new approach to tackle malaria transmission, especially in regions where traditional methods have become less effective.
The Unitaid-funded BOHEMIA project (Broad One Health Endectocide-based Malaria Intervention in Africa) conducted two Mass Drug Administration (MDA) trials in the high-burden malaria regions: Kwale County (Kenya) and Mopeia district (Mozambique). The trials assessed the safety and efficacy of a single monthly dose of ivermectin (400 mcg/kg) given for three consecutive months at the start of the rainy season in reducing malaria transmission. In Kenya, the intervention targeted children aged 5–15, while in Mozambique it focused on children under five.
In Kwale County, Kenya, children who received ivermectin experienced a 26% reduction in malaria infection incidence compared to those who received albendazole, the control drug used in the study. The trial involved over 20,000 participants and more than 56,000 treatments, demonstrating that ivermectin significantly reduced malaria infection rates—particularly among children living further from cluster borders or in areas where drug distribution was more efficient. Moreover, the safety profile of ivermectin was favourable, with no severe drug-related adverse events and only mild, transient side effects already seen with ivermectin in campaigns against neglected tropical diseases.
“We are thrilled with these results”, says Carlos Chaccour, co-principal investigator of the BOHEMIA project and ISGlobal researcher at the time of the study. “Ivermectin has shown great promise in reducing malaria transmission and could complement existing control measures. With continued research, ivermectin MDA could become an effective tool for malaria control and even contribute to elimination efforts,” Chaccour, who is now a researcher at the Navarra Centre for International Development at the University of Navarra, adds.
“These results align with the World Health Organization’s (WHO) criteria for new vector control tools”, states Joseph Mwangangi, from the KEMRI-Wellcome Trust Research Programme. “The findings suggest that ivermectin MDA could be a valuable complementary strategy for malaria control, particularly in areas where mosquito resistance to insecticides is a growing concern”, adds Marta Maia, BOHEMIA’s lead entomologist from the University of Oxford.
In contrast, the implementation of the Mozambique trial in the rural district of Mopeia faced severe disruptions due to Cyclone Gombe (2022) and a subsequent cholera outbreak, which significantly disrupted operations. “One of the most important lessons we learned from the trial in Mopeia is that strong community engagement is essential,” states Francisco Saúte, director of the Manhiça Health Research Centre (CISM). “Building trust with local communities and fostering close collaboration with the Health Ministry, National Malaria Control Program, and local authorities was key to ensuring acceptance of the ivermectin MDA.”
In addition to reducing malaria transmission, ivermectin MDA offers significant collateral benefits. The BOHEMIA team found an important reduction in the prevalence of skin infestations such as scabies and head lice in the ivermectin group in Mozambique, and the community reported a major reduction in bed bugs in Kenya. These effects are particularly valuable when ivermectin is integrated into existing delivery systems, maximising its impact on public health.
The study is part of a larger global effort to assess ivermectin’s potential in malaria control. The findings have been reviewed by the WHO vector control advisory group, which concluded that the study had demonstrated impact and recommended further studies. Findings were also shared with national health authorities as they evaluate the potential inclusion of ivermectin in malaria control programmes.
“This research has the potential to shape the future of malaria prevention, particularly in endemic areas where existing tools are failing”, concludes Regina Rabinovich, BOHEMIA PI and Director of ISGlobal’s Malaria Elimination Initiative. “With its novel mechanism of action and proven safety profile, ivermectin could offer a new approach using a well-known, safe drug that can add to the effect of other mosquito control tools available today.”
Reference:
Carlos Chaccour, Ivermectin to Control Malaria — A Cluster-Randomized Trial, New England Journal of Medicine, DOI: 10.1056/NEJMoa2411262.
Powered by WPeMatico

A new study published in the Journal of American Medical Association showed that daily application of full-body emollients during the first 9 weeks of life significantly reduced the risk of developing atopic dermatitis by the age of 2 years.
Children with atopic dermatitis (AD) face a worldwide health burden and are at risk for food allergies and asthma. Emollient intervention for primary AD prevention in infants not chosen for risk has not been well studied. In order to ascertain if emollient intervention lowers the incidence of AD by the age of 24 months among babies who were not chosen for risk, Eric Simpson and team carried out this study.
A total of 1,247 infant-parent pairs were selected from 25 community-based pediatric and family medicine clinics that are part of four statewide practice-based research networks to participate in a randomized, decentralized pragmatic clinical study. Recruitment of participants took place between July 2018 and February 2021, and follow-up was finished in February 2023.
Dyads were randomly assigned to either a control group that did not use emollients or a full-body emollient application group that began using moisturizers daily by the age of 9 weeks. By the time the kid was 24 months old, the main result was a doctor’s diagnosis of AD noted in their medical file. In order to record adverse effects and notify the team if an AD diagnosis had been established, participants filled out computerized surveys every 3 months.
At randomization, the mean (SD) age of 1247 newborns was 23.9 (16.3) days, with 553 (44.3%) of them being female. With a relative risk (RR) of 0.84 (95% CI, 0.73-0.97; P =.02), the cumulative incidence of AD at 24 months was 36.1% (SE, 2.1) in the daily moisturizer group and 43.0% (SE 2.1) in the control group. The magnitude of the effect was greater in the population that was not at high risk of AD (RR, 0.75; 95% CI, 0.60-0.90; P =.01).
The addition of a dog in the home substantially altered the protective effect (RR, 0.68; 95% CI, 0.50-0.90; P =.01). Skin-related adverse events did not differ across groups. Overall, the cumulative incidence of AD at age 24 months was found to be decreased by daily emollient application starting before age 9 weeks in a representative US population that was not selected for risk, according to this randomized clinical trial.
Source:
Simpson, E. L., Michaels, L. C., Ramsey, K., Fagnan, L. J., Nease, D. E., Henningfield, M., Dolor, R. J., Lapidus, J., Martinez-Ziegenfuss, X., Vu, A., Ferrara, L., Zuckerman, K. E., Morris, C. D., Williams, H. C., & CASCADE Consortium. (2025). Emollients to prevent pediatric eczema: A randomized clinical trial: A randomized clinical trial. JAMA Dermatology (Chicago, Ill.). https://doi.org/10.1001/jamadermatol.2025.2357
Powered by WPeMatico
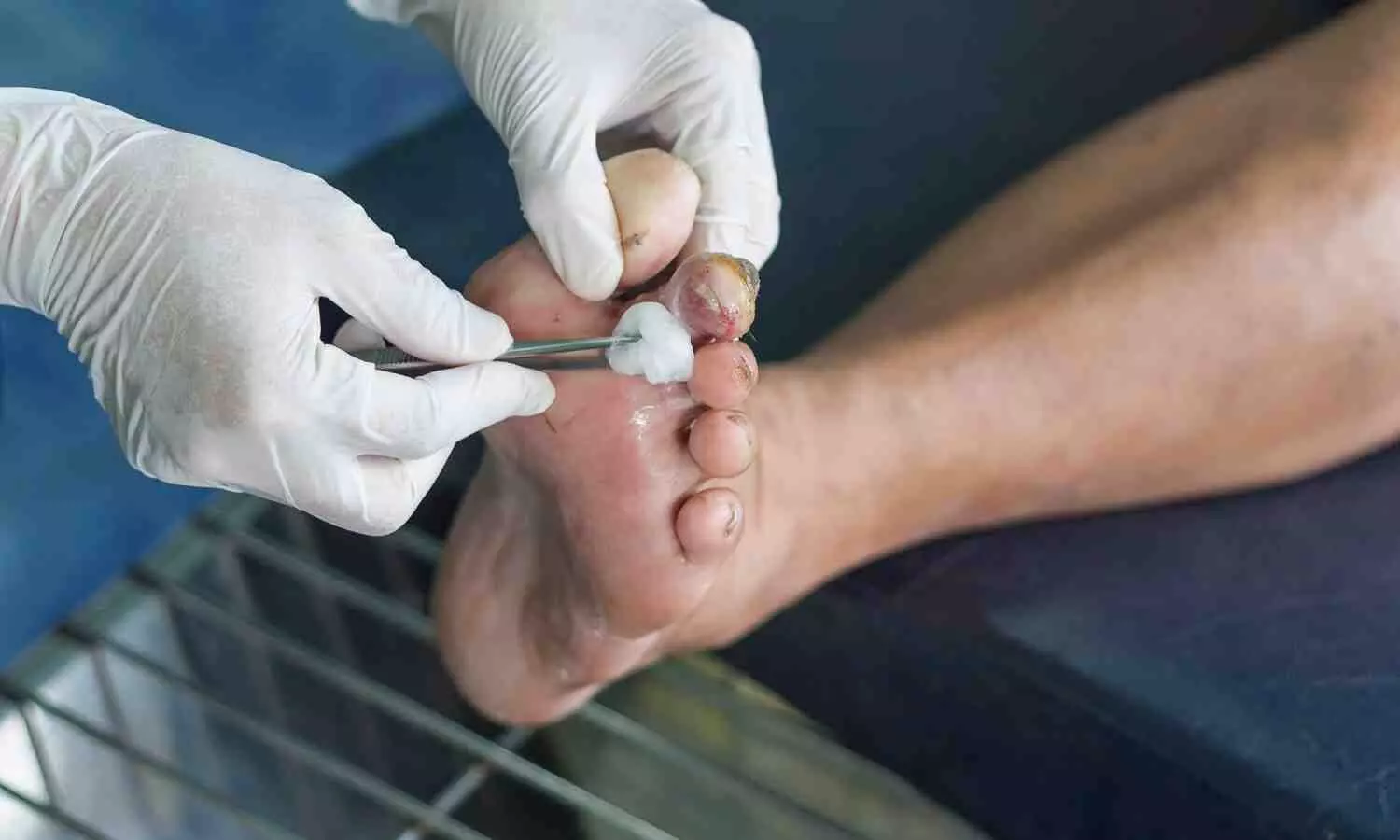
A study published in Diabetes Care has found that transepidermal water loss (TEWL) is a strong predictor of diabetic foot ulcer recurrence. Involving over 400 adults, the study revealed that individuals with high TEWL were 2.7 times more likely to experience ulcer recurrence compared to those with low TEWL.
A research team funded by the National Institutes of Health (NIH) has identified a diagnostic aid that has the potential to accurately predict the recurrence of diabetic foot ulcers that appear to be fully healed. By measuring the skin’s barrier function through a process known as trans-epidermal water loss, or TEWL, scientists were able to determine which wounds were more likely to reopen. TEWL measurements are a major factor in burn care, where deep layers of the skin are often damaged. The findings suggest that full restoration of skin barrier function should be incorporated into existing wound treatment standards to ensure complete wound closure and to better identify patients at risk of wound recurrence.
“This study is an important initial step to give clinicians treating diabetic foot ulcers a reliable diagnostic aid for the first time to assess an individual’s risk of ulcer recurrence,” said Teresa Jones, M.D. program director for the Division of Diabetes, Endocrinology, & Metabolic Diseases at NIH’s National Institute of Diabetes, Digestive and Kidney Diseases (NIDDK). “Foot ulcers are such a confounding issue with diabetes and being able to determine which wounds are at highest risk for recurrence could save many lives and limbs.”
Scientists, working together through the NIDDK Diabetic Foot Consortium, evaluated over 400 study participants who had a diabetic foot ulcer that visually appeared to be closed or healed. They measured TEWL at the site of the foot ulcer and found that 35% of participants with high TEWL (more water loss) reported a wound recurrence by 16 weeks, compared to just 17% for those with low TEWL (less water loss). Participants with higher TEWL were 2.7 times more likely to experience a wound recurrence than participants with low TEWL.
Diabetic foot ulcers are a major complication of diabetes where a break in the skin of the foot is often unnoticed by a patient due to nerve damage, known as neuropathy. They are the leading cause of non-traumatic lower-limb amputations, and untreated or unhealed ulcers significantly increase the risk of death. Wounds that appear to be healed on the surface may not be fully closed below the superficial surface of the skin, hampering the effectiveness of the skin’s barrier function to keep in water and keep out pathogens, such as bacteria.
Reference:
Chandan K. Sen, Gayle M. Gordillo, Sashwati Roy, Jordan Jahnke, Mithun Sinha, Lava Timsina, Shomita S. Mathew-Steiner, Michael S. Conte, Crystal Holmes, Teresa L.Z. Jones, Rodica Pop-Busui, Giselle Kolenic, Cathie Spino, Geoffrey C. Gurtner, NIDDK Diabetic Foot Consortium TEWL Study; High Transepidermal Water Loss at the Site of Wound Closure Is Associated With Increased Recurrence of Diabetic Foot Ulcers: The NIDDK Diabetic Foot Consortium TEWL Study. Diabetes Care 2025; dc250300. https://doi.org/10.2337/dc25-0300
Powered by WPeMatico

A new study published in the journal of Clinical Gastroenterology and Hepatology showed that the possibility of vitamin D supplementation as an inexpensive adjuvant in the treatment of inflammatory bowel disease (IBD) was supported by its association with a decrease in IBD-related ED visits, hospitalizations, and corticosteroid use.
Providing vitamin D is undoubtedly the most practical and efficient method to break the positive feedback loop that has been suggested to exist between vitamin D deficiency, the inflammatory process, and IBD. Intestinal dysbiosis, immunological disorders, genetic predisposition, and environmental risk factors are all linked to the pathophysiology of IBD. This study assessed the practical effects of vitamin D supplementation on hospitalizations, ED visits, and corticosteroid use in IBD patients.
The patients with IBD who were seen in the nationwide Veterans Health Administration system between 2000 and 2023 were the subjects of this retrospective cohort research. All individuals who received a vitamin D lab test within the previous 3 months without a prescription for vitamin D were included. Before and after the 25-hydroxyvitamin D assay, this research compared the vitamin D-treated and untreated groups using a quasi-experimental design with difference-in-differences analysis. Inverse probability weighting (IPW) regression and regression discontinuity design (RDD) were employed in sensitivity studies to validate the findings of the initial study.
The median 25-hydroxyvitamin D level was 23 ng/mL among 5,021 IBD patients (median age 63; 89% male; 58% ulcerative colitis, 39% Crohn’s disease, and 3% indeterminate colitis), and 41% of them took vitamin D supplements. IBD-related ED visits decreased by 2.17% (34.4% RRR, p=0.007), hospitalizations decreased by 2.64% (53.18% relative risk reduction (RRR), p=0.003), and corticosteroid prescriptions decreased by 1.29% (25.13% RRR, p=0.066) when vitamin D supplementation was taken. The RDD and inverse probability weighted regression analysis produced comparable findings.
Overall, supplementation was associated with better long-term results in this recent study which included 5,021 patients with vitamin D deficiency and IBD. Supplementing with vitamin D reduced the use of corticosteroids by 25%, hospitalizations by 53%, and ED visits by 34%. These advantages were noted in both Crohn’s disease and ulcerative colitis, suggesting that it might be used as a supplement to IBD treatment.
Reference:
Sninsky, J. A., Sansgiry, S., Taylor, T., Perrin, M., Kanwal, F., & Hou, J. K. (2025). The real-world impact of vitamin D supplementation on inflammatory bowel disease clinical outcomes. Clinical Gastroenterology and Hepatology: The Official Clinical Practice Journal of the American Gastroenterological Association. https://doi.org/10.1016/j.cgh.2025.07.013
Powered by WPeMatico
Acute myeloid leukemia (AML) is an aggressive cancer affecting the blood and bone marrow that progresses rapidly, making immediate treatment essential. While chemotherapy and targeted drugs have improved outcomes for some patients, many forms of AML remain resistant to treatment, and relapses are common.
A new study sheds light on why certain types of AML are so challenging to treat and how outcomes for patients might be improved. Researchers from Japan, including scientists from Chiba University, have discovered that an epigenetic enzyme called SETD1B plays a critical role in supporting the growth of a particularly aggressive form of AML—especially in patients with the Fms-like tyrosine kinase-3 internal tandem duplication (FLT3-ITD) mutation, a genetic change associated with poor prognosis and high relapse rates. By targeting SETD1B, the researchers believe, it may be possible to develop more effective treatments that prevent leukemia cells from multiplying.
The study was led by Associate Professor Takayuki Hoshii and included Dr. Shintaro Izumi and Professor Atsushi Kaneda from the Graduate School of Medicine, Chiba University, Japan. The findings were published online in the journal Leukemia on May 8, 2025.
“Patients with AML, especially with the FLT3-ITD mutation, often respond poorly to current therapies. Our findings are that the epigenetic regulator SETD1B protein supports aggressive cell proliferation in AML by promoting oncogenic MYC expression,” says Dr. Hoshii.
Previous research has shown that FLT3-ITD mutations are detected in patients with mixed-lineage leukemia-rearranged (MLL-r) AML, a subtype of leukemia characterized by genetic abnormalities. MLL-r AML cells exhibit high levels of histone H3 lysine 4 trimethylation (H3K4me3), an epigenetic modification that affects which genes are turned on or off without changing the DNA sequence. Though the link between MLL-r AML and H3K4me3 has been established, the association between FLT3-ITD and H3K4me3 remains unclear.
Using advanced genetic screening tools, the team identified Setd1b as the gene responsible for producing a protein that adds methyl groups to histone H3 at lysine 4, resulting in the H3K4me3 epigenetic modification, and upregulation of Myc genes, which plays a critical role in regulating cell growth, division, and metabolism and can contribute to cancer development if altered.
To understand the role of SETD1B, the researchers performed CRISPR screening and then genetically engineered leukemia cells with altered versions of SETD1B, specifically deleting its catalytic domain, which is the active part of the protein responsible for the epigenetic modification of the DNA. They then used RNA sequencing and chromatin mapping to identify which genes and pathways were most affected.
Their experiments revealed that removing SETD1B’s catalytic domain significantly slowed cancer growth, particularly in leukemia cells with FLT3-ITD or NrasG12D mutations. It also caused a drop in the activity of genes in the MYC pathway. This suggests that without the epigenetic function of SETD1B, MYC cannot remain fully active, weakening the ability of the cancer cells to grow and divide.
“The breadth of H3K4me3 is crucial for transcriptional consistency, and MYC expression appears highly dependent on both the quality and quantity of transcriptional elongation,” says Dr. Hoshii. “Understanding SETD1B’s role in maintaining this epigenetic mark is critical for developing biomarkers and therapies for leukemia subtypes and other MYC-driven cancers.”
Interestingly, when the researchers reintroduced the Myc gene back into leukemia cells lacking SETD1B, the cancer cells began to grow again, but only partially, suggesting that SETD1B plays a broader role in MYC’s cancer-promoting activity.
This discovery paves the way for new treatment strategies. By targeting SETD1B or its epigenetic functions, scientists may be able to develop therapies that are especially effective for patients with FLT3-ITD mutations. As a possible next step, the researchers point to Chaetocin, an existing compound known to inhibit enzymes related to SETD1B. This could serve as a foundation for developing more selective SETD1B-targeted drugs. Measuring SETD1B activity in patients might also help doctors predict who would benefit most from these therapies.
As the search for better treatments continues, this study reveals how targeting the cancer’s epigenetic machinery could provide a new strategy to treat the disease, giving hope to patients with aggressive forms of AML.
Reference:
Izumi, S., Ohtani, K., Matsumoto, M. et al. Regulation of H3K4me3 breadth and MYC expression by the SETD1B catalytic domain in MLL-rearranged leukemia. Leukemia 39, 1627–1639 (2025). https://doi.org/10.1038/s41375-025-02638-y.
Powered by WPeMatico
