Genetic testing reduces risks from chemotherapy for gastrointestinal cancer patients: Study

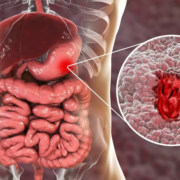
For some patients with gastrointestinal (GI) cancers like colorectal and pancreatic cancer, chemotherapy can cause severe, sometimes life-threatening side effects in those who carry certain genetic variants that can impact how their bodies process the drugs used to treat their disease. Testing for variants in two genes before starting chemotherapy can significantly improve patient safety by providing physicians with information to help tailor doses, according to new research from the Perelman School of Medicine at the University of Pennsylvania. Those who were found to have one of the genes had half as many side effects in half, as compared to patients with the genes that were given standard doses without testing, according to results published today in JCO Precision Oncology.
“For too long, the U.S. lagged behind Europe in adopting genetic testing for chemotherapy dosing, but our study shows it’s not only feasible but also critical for patient safety,” said the study’s lead author, Sony Tuteja, PharmD, MS, Director of Pharmacogenomics in the Penn Medicine Center for Genomic Medine, a research assistant professor of Translational Medicine and Human Genetics. “With up to 1,300 deaths in the U.S. each year due to side effects from one of the most common forms of chemotherapy drugs, we’ve worked to make testing fast and actionable, getting results in about a week to help doctors make safer treatment decisions.”
Nearly 290,000 Americans are diagnosed with gastrointestinal cancers each year – including colorectal cancer, the third most common cancer diagnosis in the nation. Current chemotherapy protocols use standard dosing standards that don’t account for genetic differences in how patients process these drugs.
Genetic Variants Guide Safer Chemotherapy
The study focused on variants in two genes: DPYD and UGT1A1. The DPYD gene produces an enzyme that helps the liver break down drugs like fluoropyrimidines, which are commonly used in gastrointestinal cancer treatment. About 5 to 8% of people carry DPYD variants that hinder the body’s ability to process fluoropyrimidine chemotherapy drugs, causing them to build up to harmful levels, which can lead to serious side effects like reduced blood cell production, mouth sores, or hand-foot syndrome. Similarly, the UGT1A1 gene affects how the body processes irinotecan, another key chemotherapy drug often used to treat GI cancers. Variants in UGT1A1 can lead to the body processing the drug too slowly, increasing the risk of severe diarrhea or low white blood cell counts. By identifying these variants, doctors can lower chemotherapy doses to prevent harmful side effects without compromising treatment effectiveness.
The study enrolled 517 GI cancer patients at three cancer care sites in the University of Pennsylvania Health System who were scheduled to begin chemotherapy treatment with fluoropyrimidine or irinotecan. A group of 288 received blood tests to check for DPYD and UGT1A1 variants. Among 16 patients who were found to have genetic variants and received tailored dose reductions based on test results, 38% experienced severe treatment-related adverse events. In comparison, 65% of 17 patients with the genetic variants from a biobank group who received standard doses without prior testing experienced the serious side effects. The tested group also saw a significantly lower need to change treatment dosage and frequency (38% vs. 76%) and fewer treatment discontinuations (31% vs. 47%), highlighting the potential of precision medicine to enhance patient safety and outcomes.
Powered by WPeMatico