Medically tailored meals improve nutrition, reduce readmissions for heart failure patients
Powered by WPeMatico
Powered by WPeMatico

North Chicago, Ill.: AbbVie has announced submission of a Biologics License Application (BLA) to the U.S. Food and Drug Administration (FDA) for trenibotulinumtoxinE (TrenibotE) for the treatment of moderate to severe glabellar lines.
“The submission provides evidence of TrenibotE’s differentiated clinical profile to offer patients an opportunity to experience a faster onset and shorter treatment duration as an introduction to a neurotoxin,” said Darin Messina, Ph.D., senior vice president, aesthetics R&D, AbbVie. “TrenibotE has the potential to transform the aesthetic toxin treatment landscape for new patients interested in the facial aesthetics category.”
“New patients wanting to experience the aesthetic benefits of a neurotoxin cite “fear of looking unnatural” as a barrier to initiating neurotoxin use for aesthetic indications. If approved, TrenibotE will be the first serotype E neurotoxin offering patients the opportunity to experience a neurotoxin with rapid clinical effect for a shorter duration of time as a trial before getting treatment with BOTOX Cosmetic,” the Cmpany stated in a recent releae.
The BLA submission is supported by data from over 2,100 patients treated with TrenibotE in the clinical program, which included two pivotal Phase 3 clinical studies evaluating TrenibotE for the treatment of moderate to severe glabellar lines (M21-500 and M21-508) and a Phase 3 open-label safety study (M21-509). All primary and secondary endpoints of the Phase 3 studies were met, with a rapid onset of action as early as 8 hours after drug administration (the earliest assessment time) and observed efficacy duration for 2-3 weeks. Treatment-emergent adverse events for TrenibotE were similar to placebo, both as a single treatment and up to three consecutive treatments. Topline data from the Phase 3 pivotal studies were previously shared.
“Concern about an unnatural outcome remains a significant barrier for many patients considering medical aesthetics treatment,” said Cheryl Burgess, MD, FAAD, lead clinical investigator for one of the Phase 3 studies. “Treatment with a product offering rapid onset of effect and short duration of action could help address this barrier and empower confidence for patients exploring their aesthetics treatment journey with innovation from the makers of BOTOX Cosmetic.”
Powered by WPeMatico

New Delhi: A Delhi court has granted bail to a laundry operator at the Atal Bihari Vajpayee Institute of Medical Sciences and Hospital, who was allegedly caught red-handed while accepting a bribe of Rs 8000 from the complainant, during a trap laid by the Central Bureau of Investigation (CBI).
Special Judge for CBI Jyoti Kler after hearing the arguments, said, “Accused was arrested in this case on 27.03.2025. He was remanded to JC on 28.03.2025. On Court’s question, IO has disclosed that the complainant and other independent witnesses, except one, have already been examined by him. Recovery has already been effected. Investigation is about to be over. The accused is no longer required for custodial interrogation”.
The court said, “Considering the aforesaid submissions of the IO, period of custody of the accused, stage of investigation, period likely to be spent in conducting the trial and other facts & circumstances in totality, the accused, namely, Sh. Surjeet Kumar, is admitted to bail on furnishing of bail bond in the sum of Rs.1,00,000/- with one surety in the like amount,” news agency UNI reported.
Also Read:Stent Bribery Case Exposed at RML Hospital, Two Senior Cardiologists Arrested by CBI
The court, while granting bail, laid down certain conditions that the accused shall attend the Court regularly during the trial and appear before the IO for the purpose of further investigation, as and when so directed.
He shall not commit an offence similar to the offence of which he is accused/suspect, or of the commission of which he is suspected and shall not directly or indirectly make any inducement, threat or promise to any person acquainted with the facts of the case so as to dissuade him from disclosing such facts to the Court or to any police officer. He shall not tamper with the evidence directly or indirectly and shall not leave the territory of India without seeking prior permission of the Court and shall place on record a copy of his passport within five days of his release from the custody.
Counsel for the accused has moved the bail application with the submission that he has been falsely implicated. He has no concern with the RML Hospital. He was working as a laundry operator in the Atal Bihari Vajpayee Institute of Medical Sciences and Hospital.
Sr. PP for the CBI has opposed the bail application by submitting that the accused was caught red handed while accepting bribe in the sum of Rs.8,000/- from the complainant, during a trap laid down by the CBI. He said the allegations are serious in nature & charge sheet is yet to be filed, so the bail application of accused may kindly be dismissed at this stage.
Also Read:Sudden death of senior resident doctor while on duty at RML Hospital shocks colleagues
Powered by WPeMatico

New Delhi- Through a recent notice, the National Medical Commission (NMC) has directed the medical colleges to submit Additional Information in the Annual Declaration Form for Annual Renewal for the AY 2025-26 on the NMC Portal.
The process of assessment for the annual renewal of all medical colleges recognised by NMC is currently underway. As per the public notice released by the NMC, it has been observed that certain colleges have not filled in the required details related to cadavers under the heading ‘Clinical Load – Anatomy Department’. Therefore, it is to be noted that the information related to the number of cadavers handled by each medical college is mandatory.
Additionally, all medical colleges/institutes are required to provide the Number of Major Operation Theatres (OTs) and the Number of Minor Operation Theatres (OTs) details in the Annual Declaration Form. The relevant fields to submit this information have been created under the heading ‘Hospital Details’ in the Annual Declaration Form. To facilitate the submission of the above details, the NMC Portal opened from 25 April 2025.
The National Medical Commission (NMC) is going to close the facility for submitting additional information by 28 April 2025. In this regard, the NMC has issued a letter to all the directors, principals and deans requesting them to take necessary action and adhere to the deadline.
As per the letters, the Undergraduate Medical Education Board (UGMEB) is requested to submit the additional information regarding cadavers/major and minor OTs in the annual declaration form for annual renewal by 28 April 2025.
The process of assessment for annual renewal of all medical colleges
recognized by NMC is currently underway. However, it has been observed that
certain colleges have not filled in the required details related to cadavers
under the heading ‘Clinical Load – Anatomy Department’. Kindly note that the
information related to number of cadavers handled by each medical college is
mandatory information.Additionally, all medical colleges/institutes are required to provide the
following details in the Annual Declaration Form:
a. Number of Major Operation Theatres (OTs)
b. Number of Minor Operation Theatres (OTs)The relevant fields to submit this information have been created under the heading
‘Hospital Details’ in the Annual Declaration Form.To facilitate submission of the above details, theNMC Portal will remain open from 25.04.2025 to 28.04.2025. The concerned medical colleges/institutes are hereby informed to furnish all the above information within the stipulated time.
The uploading of Annual Declaration details/data by respective medical colleges/institutions on the NMC Portal, as mandated under the NMC Act, 2019 and relevant regulations issued by the Commission from time to time.
To view the letter, click the link below
Powered by WPeMatico

Bhadrak: In a tragic incident, a 54-year-old woman patient allegedly died of suspected suffocation on Thursday after being stuck in an elevator in which she was being shifted to a ward in Bhadrak District Headquarters Hospital here developed a technical snag and got stranded for some time, officials said.
Hospital sources said that the woman was brought to the hospital in an ambulance after she fell ill. She had respiratory problems. After receiving preliminary treatment, she was being shifted to the medicine ward when the mishap occurred, news agency PTI reported.
Also Read:Stuck in lift for an hour: Woman dies after childbirth as Meerut Hospital lift crashes
“The patient identified as Minati Parida of Narasinghapur village was being shifted to the medicine ward of the hospital. Due to the malfunction of the elevator the woman got trapped. She is suspected to have died of suffocation. The reason for the death will be ascertained only after the autopsy,” Chief District Medical Officer (CDMO), Bhadrak, Sudhansu Sekhar Bal told reporters.
An investigation has been initiated to ascertain the exact cause of the elevator failure, the CDMO said.
Powered by WPeMatico

New Delhi: To strengthen disease surveillance and improve diagnostic infrastructure, the Delhi Health Department has identified 11 district hospitals to host Integrated Public Health Laboratories (IPHL). These labs will serve as a vital link between primary health centres, sub-centres, and more advanced diagnostic support systems across the city.
The initiative, designed to bolster both routine diagnostics and outbreak response capabilities, is expected to begin operations within three months of receiving final approval from senior authorities, according to a senior health department official.
Each IPHL will deliver a wide range of diagnostic services, including pathological, biochemical, and haematological tests.
Also Read: Strengthening Public Health Infrastructure to Prevent Future Pandemics: Health Minister
The procurement of equipment will be aligned with specific requirements outlined in the 2021 Integrated Public Health Laboratories (IPHL) guidelines. Essential devices include an automated coagulometer, an automated ESR analyzer, a centrifuge, a binocular microscope, a fluorescent microscope, a real-time PCR machine, and an automated blood culture system.
A health official stated that, since district hospitals already possess various testing devices, the existing infrastructure will be utilized before acquiring any new equipment. According to the news reports, the proposed district hospitals where the labs will be set up include Lal Bahadur Shastri Hospital in east, Maharishi Valmiki Hospital in north, Guru Gobind Singh Govt Hospital in west, Pt Madan Mohan Malaviya Hospital in south, Ambedkar Nagar Hospital in south-east, Indira Gandhi Hospital in south-west, and Rajiv Gandhi Super Specialty Hospital in Shahdara.
As per the Indian Public Health Standards (IPHS), each lab will operate 24/7 and must include three specialists (pathologist, microbiologist, and biochemist), 11 laboratory technicians across three shifts, plus data entry operators, cleaning staff, housekeeping, and security personnel.
Also Read: Himachal allocates Rs 193.75 crore for 2 New Critical Care Blocks
Officials emphasized that integrating lab services will maximize resource efficiency, avoid duplication, and enhance patient care. With multi-disease testing capabilities, IPHLs will improve the city’s readiness for emerging health threats and enable rapid, accurate reporting to public health surveillance systems.
Beyond diagnostics, the IPHLs will function as critical components of Delhi’s public health response system, analyzing not only clinical specimens but also environmental samples like water, food, and air, especially during health emergencies and disease outbreaks.
IPHLs also provide diagnostic services for both communicable and non-communicable diseases, including tests under national health programmes for tuberculosis (TB), HIV, malaria, viral hepatitis, and other conditions that require Biosafety Level 2 laboratory standards.
Samples collected at IPHLs will typically fall into two main categories: blood and compartmental specimens (e.g., nasal swabs, sputum, saliva, urine). Transportation protocols will vary depending on the sample type and specific test requirements.
Powered by WPeMatico

An Instagram post claims that Rubbing a Tomato With Sugar Brightens the skin. The claim is MISLEADING.
In an Instagram post, it is claimed that Rubbing a Tomato With Sugar Brightens the skin. The post by user hindi.beauty.tips says, “Rubbing sugar on a tomato and applying it to the face helps remove dead skin cells and brighten the skin.”
The post can be accessed here.
The claim by user is MISLEADING. Tomato application and consumption might offer skin benefits such as improved brightness and elasticity and topical application of tomato with sugar could aid in exfoliation. However, no scientific evidence or medical consensus supports the claim that applying tomatoes topically can remove dead skin cells or visibly brighten the skin n.
Tomatoes are among the most commonly consumed foods worldwide, valued for their abundance of vital nutrients and powerful antioxidants. They are a good source of minerals, vitamins, proteins, and essential amino acids like leucine, threonine, and lysine. Additionally, they contain beneficial monounsaturated fatty acids such as linoleic and linolenic acids, carotenoids like lycopene and β-carotene, and phytosterols including β-sitosterol, campesterol, and stigmasterol. Lycopene, the primary carotenoid in tomatoes, has been associated with a lower risk of various health issues, including cancer, heart disease, cognitive disorders, and osteoporosis. The antioxidant compounds in tomatoes help safeguard the body by neutralizing reactive oxygen species, thus minimizing oxidative damage to key cellular components like lipids, proteins, and DNA. As a result, incorporating tomatoes into the diet may boost antioxidant defenses and reduce the risk of conditions related to oxidative stress.
Sugar is a type of carbohydrate that includes naturally occurring forms like glucose and fructose, found in fruits and vegetables, and refined forms like sucrose and high-fructose corn syrup (HFCS), commonly added to processed foods. While natural sugars are essential for energy and part of whole foods, refined sugars are highly processed and not found in nature except in rare cases like honey. These added sugars are a recent addition in human evolution and are now present in nearly 75% of packaged foods, contributing to various health concerns when consumed in excess.
Tomato application and consumption may help improve skin brightness and elasticity. Hence, the claim by the user is MISLEADING.
Tomato extracts are increasingly recognized in dermatological research for their skin-enhancing properties. A study on tomato seed extract by Mrs. Vaishali Sorte et. al. found that they exhibit multiple dermatological benefits, including anti-aging effects, prevention of hyperpigmentation, regulation of sebum (oil) production, and improvement in skin brightness and pore tightness.
Tomatoes, rich in antioxidants like lycopene, have long been studied for their potential skin benefits. A recent study published in the Journal of Dermatologic Science and Cosmetic Technology evaluated that continuous intake of the tomato extract formulation over eight weeks significantly improved skin hydration and visibly brightened skin, likely through its antioxidant mechanism.
Tomato consumption has also been associated with skin benefits like enhanced skin elasticity. A study published in the Journal of Cosmetics, Dermatological Sciences and Applications found that daily consumption of tomato seed extract may help maintain and improve facial skin elasticity.
Hence, the claim is MISLEADING.

Dr. Varsha Rangari, Sr.Consultant – Dermatologist & Cosmetologist, Aditya Birla Memorial Hospital in a conversation with The Medical Dialogues Fact Check Team, said, “Tomato contains natural acids like citric and malic acid that can mildly exfoliate the skin, while sugar acts as a physical exfoliant. When gently rubbed, this combination can temporarily brighten the complexion. However, there is no scientific evidence that it’s a substitute for scientifically formulated skincare products and should be used with caution, especially on sensitive skin. Also, one has to be careful about allergies to any of the ingredients.”

Dr. Madhur Basude, Consultant – Dermatologist, Aditya Birla Memorial Hospital, further said, “Using a mix of tomato and sugar as a DIY scrub can offer a temporary glow by sloughing off dead skin cells—thanks to the fruit’s natural acids and sugar’s exfoliating texture. While it might feel refreshing, it’s important to remember that not all skin types react the same way. Always patch test first, as natural ingredients can still cause irritation or allergic reactions. Further, there is no scientific evidence or medical consensus that it can be used to remove dead skin cells or brighten skin.”
The claim that rubbing a tomato with sugar brightens the skin is misleading. While applying and consuming tomatoes may support skin brightness and elasticity due to their antioxidant content, and rubbing tomato with sugar may have an exfoliating effect, there is no scientific evidence confirming that this topical mixture brightens the skin.
Hence, the claim is MISLEADING.
Powered by WPeMatico

Sweden: A recent study published in Diabetes, Obesity and Metabolism has identified a potential biological marker tied to mental health in patients with newly diagnosed type 2 diabetes (T2D).
The study revealed that individuals with newly diagnosed type 2 diabetes and low levels of soluble neuropilin-1 (sNRP-1) were more likely to experience depression. Notably, 45% of patients with depression had reduced sNRP-1 levels compared to just 22% among those without depressive symptoms, underscoring a strong link between low sNRP-1 and the presence of depression.
The neuropilin-1 (NRP-1) receptor is a crucial transmembrane glycoprotein that plays a significant role in the development of the nervous and cardiovascular systems, immune modulation, and potentially in diabetes-related complications. Its widespread presence in tissues such as the brain, heart, kidneys, retina, pancreatic beta cells, and adipose tissue macrophages suggests its broad physiological impact. Given its biological relevance, E. O. Melin PhD, Diabetes Research Laboratory, Biomedical Center, Lund University, Lund, Sweden, and colleagues aimed to investigate the relationship between sNRP-1 levels and depression in individuals with newly diagnosed type 2 diabetes, highlighting a potential biomarker for early mental health assessment in this population.
For this purpose, the researchers conducted a multicentre, cross-sectional study involving adults with newly diagnosed type 2 diabetes confirmed through blood tests. They collected data on several factors, including sex, levels of soluble neuropilin-1 (with low sNRP-1 defined as less than 226 ng/mL), depression and anxiety assessed using psychological tests, use of antidepressants, body mass index (BMI), haemoglobin A1c, C-peptide levels, and history of cardiovascular disease. To better understand the associations, they performed multiple regression analyses, using depression and low sNRP-1 levels as the main outcomes.
The key findings of the study were as follows:
The findings showed that low levels of soluble neuropilin-1 were strongly linked to depression in adults with newly diagnosed type 2 diabetes. This association was evident across all age groups. Among younger patients (under 60 years), low sNRP-1 levels were also independently associated with pre-existing myocardial infarction and younger age, highlighting a more complex relationship in this subgroup. In older patients, depression and younger age within the group remained significantly linked to reduced sNRP-1 levels.
“These findings suggest that sNRP-1 may serve as a potential biomarker for identifying individuals at higher risk of depression in early-stage T2D,” the authors concluded.
Reference:
Melin EO, Thunander M, Wanby P, et al. Low levels of soluble neuropilin-1 were associated with depression in adults with newly diagnosed type 2 diabetes. Diabetes Obes Metab. 2025;1‐10. doi:10.1111/dom.16347
Powered by WPeMatico
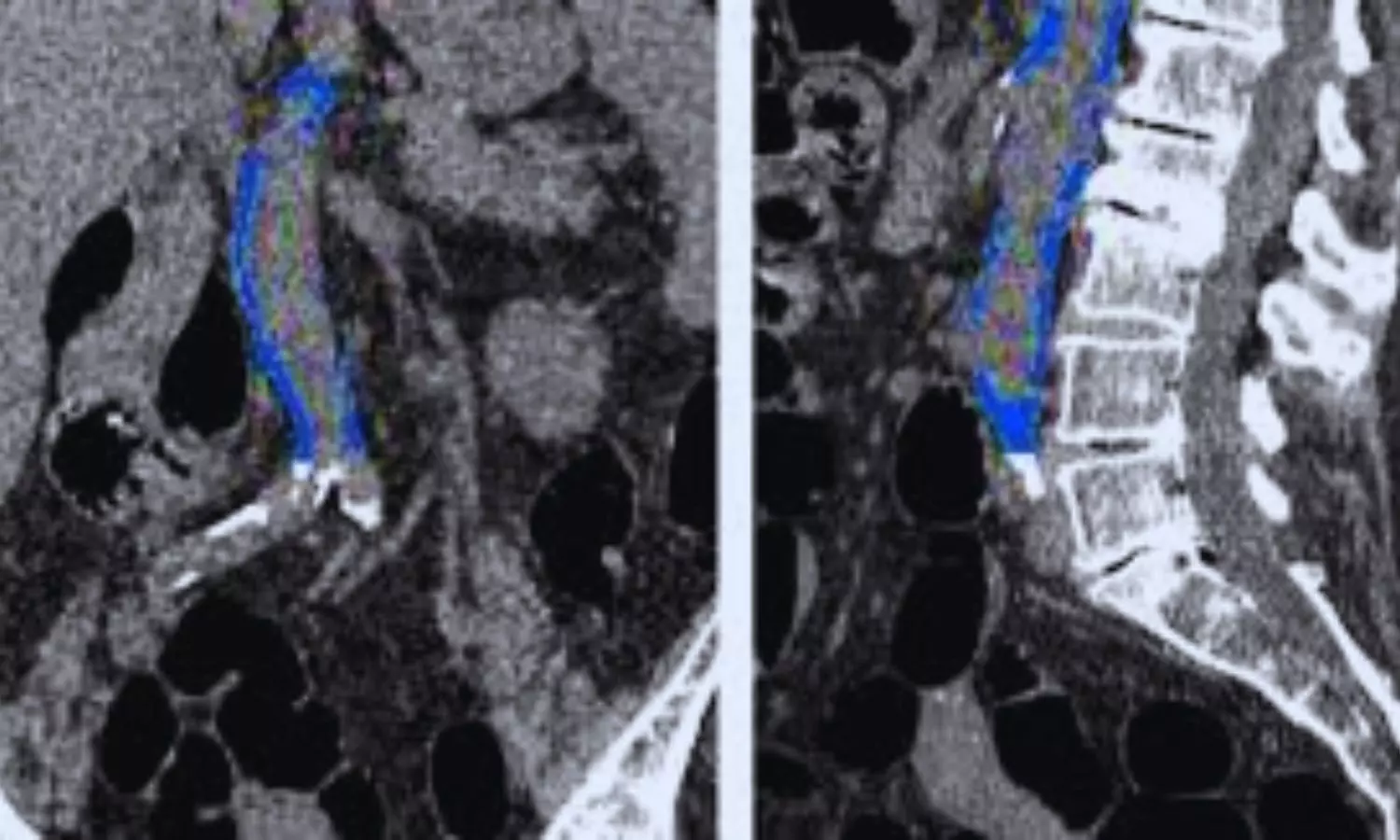
China: A new analysis of data from the National Health and Nutrition Examination Survey (NHANES) 2013–2014 has revealed a significant association between frailty and abdominal aortic calcification (AAC) in US adults aged 40 and older.
The study, published in Frontiers in Public Health, found that individuals with higher Frailty Index (FI) scores had significantly elevated AAC scores (β = 2.64). Notably, frail individuals (FI > 0.25) had a 6.39-fold increased likelihood of severe AAC (defined as a score >6). Additionally, FI Z-scores demonstrated a linear relationship with severe AAC, suggesting that the FI could be a valuable early biomarker for subclinical atherosclerosis and cardiovascular risk in aging populations.
Abdominal aortic calcification is one of the earliest indicators of atherosclerotic calcification and is vital in predicting early cardiovascular risk. Frailty, a significant clinical and public health concern, is linked to increased risks of mortality, functional decline, and loss of independence. Despite its importance, the relationship between frailty and AAC in middle-aged and older adults has not been extensively explored. To address this gap, Zhengjun Zhang, Department of Cardiology, Yinchuan, China, and colleagues analyzed data from the 2013-2014 NHANES, focusing on individuals aged 40 years and older.
For this purpose, the researchers used a 49-item model to calculate the Frailty Index and categorized participants into three groups: non-frail (FI ≤ 0.15), pre-frail (0.15 < FI ≤ 0.25), and frail (FI > 0.25). Abdominal aortic calcification was measured using dual-energy X-ray absorptiometry and quantified using Kauppila scores, with severe AAC defined as a score greater than 6. The relationship between FI and AAC was explored through multivariable logistic regression, sensitivity analyses, and smoothing curve fitting. To ensure the robustness of the findings, subgroup analyses, and interaction tests were conducted across different populations.
The study led to the following findings:
“Our study demonstrates a consistent positive correlation between the Frailty Index and abdominal aortic calcification,” the researchers concluded. They further emphasized, “The findings suggest that FI could serve as a valuable biomarker for the early detection of subclinical atherosclerosis in middle-aged and older adults in the United States.”
Reference:
Zhang, Z., Wu, P., Yang, S., Zhu, B., Chen, D., Li, X., Wang, Y., & Yan, N. The association between frailty index and abdominal aortic calcification in the middle-aged and older US adults: NHANES 2013-2014. Frontiers in Public Health, 13, 1546647. https://doi.org/10.3389/fpubh.2025.1546647
Powered by WPeMatico

Scientists from Northwestern University identified piperacillin, an FDA-approved antibiotic, as a strong candidate for treating B. burgdorferi, the bacterium behind Lyme disease, while sparing other bacteria. In mouse studies, piperacillin proved as effective as doxycycline in treating acute infection. Additionally, separate findings revealed that B. burgdorferi peptidoglycan can persist even after infection clearance, offering a possible explanation for lingering Lyme disease symptoms in humans. The study has been published in the journal Science Translational Medicine.
Lyme disease, a disease transmitted when deer ticks feed on infected animals like deer and rodents, and then bite humans, impacts nearly half a million individuals in the U.S. annually. Even in acute cases, Lyme can be devastating; but early treatment with antibiotics can prevent chronic symptoms like heart and neurological problems and arthritis from developing.
Doxycycline and other generic antibiotics wreak havoc on the microbiome, killing beneficial bacteria in the gut and causing troubling side effects even as it kills the borrelia bacteria that causes Lyme. In addition to its negative impact on the gut, doxycycline also fails to help between 10 and 20% of individuals who take it, and it is not approved for use in young children — who are at the highest risk of tick bites, and therefore, of developing Lyme.
More effective, or at least more specified, treatment options are needed as climate change extends tick seasons and Lyme becomes more prevalent.
“Powerful, broad-spectrum antibiotics that kill extracellular bacteria are seen as the most effective medication because physicians want to just kill the bacterium and don’t care how,” said Brandon L. Jutras, who led the research. “This is certainly a reasonable approach, but I think the future for Lyme disease patients is bright in that we are approaching an era of customized medicine, and we can potentially create a particular drug, or a combination to treat Lyme disease when other fail. The more we understand about the various strains and species of Lyme disease-causing Borrelia, the closer we get to a custom approach.”
Jutras is an associate professor in the microbiology-immunology department of Northwestern University Feinberg School of Medicine, and a member of Northwestern’s Center for Human Immunobiology. Jutras’s lab was recently named a Phase 3 winner in LymeX Diagnostics, the Steven & Alexandra Cohen Foundation’s $10 million competition to accelerate the development of Lyme disease diagnostics, and in 2021 he won the Bay Area Lyme Foundation Emerging Leader Award.
The authors argue that piperacillin, which has already been FDA-approved as a safe treatment for pneumonia, could also be a candidate for preemptive interventions, in which someone potentially exposed to Lyme (with a known deer tick bite) would receive a single-dose shot of the medication.
To reach the conclusion that the penicillin relative would be the most effective and targeted treatment, the team screened nearly 500 medicines in a drug library, using a molecular framework to understand potential interactions between antibiotics and the Borrelia bacteria. Once the group had a short list of potentials, they performed additional physiological, cellular and molecular tests to identify compounds that did not impact other bacteria.
They found that piperacillin exclusively interfered with the unusual cell wall synthesis pattern common to Lyme bacteria, preventing the bacteria from growing or dividing and ultimately leading to its death.
Historically, piperacillin has been administered as part of a two-drug cocktail to treat severe strep infections because strep can break down beta-lactams (piperacillin’s class of antibiotics) unless accompanied by tazobactam, which is an inhibitor of the enzyme that inactivates piperacillin. Jutras wondered if using the same two medications, rather than piperacillin alone, would be a more effective bacteria killer.
“Bacteria are clever,” Jutras said. “Strep and some other bacteria combat antibiotics by secreting beta-lactamases that inactivate piperacillin. We found the approach is totally irrelevant in the context of Lyme disease and another way that makes piperacillin more specific. Adding the beta-lactamase inhibitor doesn’t improve the therapy because Lyme Borrelia don’t produce beta-lactamase, but the cocktail does negatively impact the microbiome by becoming more broadly functional against beneficial residents.”
Lyme prevention remains a challenge-no approved human vaccine exists-and Jutras hopes his research moving forward will help with developing proactive strategies to diagnose and treat it.
Reference:
Maegan E. Gabby, Abey Bandara, L. M. Outrata, Osamudiamen Ebohon, Saadman S. Ahmad, Jules M. Dressler, Mecaila E. McClune, Rebecca N. Trimble, Lainey Mullen, Brandon L. Jutras. A high-resolution screen identifies a preexisting beta-lactam that specifically treats Lyme disease in mice. Science Translational Medicine, 2025; 17 (795) DOI: 10.1126/scitranslmed.adr9091
Powered by WPeMatico
