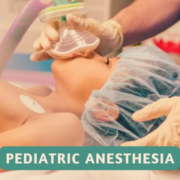
Renal disease in pediatric patients presents significant challenges, particularly in anesthetic management, due to its association with high morbidity and mortality rates. Achieving effective perioperative pain management is crucial, and pediatric regional anesthesia emerges as a valuable strategy to enhance pain control. Recent study explores the impact of ultrasound-guided supraclavicular brachial plexus block (BPB) as an adjunct to sevoflurane anesthesia during brachiobasilic arteriovenous fistula (AVF) operations in children with end-stage renal disease (ESRD).
Challenges in AVF Creation and the Role of BPB
The creation of AVF is critical for hemodialysis access, yet the procedure faces complications, including a high primary failure rate largely attributed to inadequate vessel dilation and sympathetic overflow-induced vasospasm. The implementation of BPB can induce sympathetic blockade, leading to vessel dilation and improved blood flow—key factors in ensuring successful AVF maturation.
Study Design and Objectives
In a randomized controlled trial, 60 pediatric patients with ESRD participated, divided into two groups receiving either general anesthesia with BPB or general anesthesia with saline injection. The study aimed to measure the time to AVF maturation, hemodynamic stability during surgery, the incidence of emergence agitation, and postoperative analgesia.
Results and Clinical Implications
Results indicated that patients receiving BPB demonstrated significantly improved outcomes: faster maturation of the AVF (mean of 12 weeks compared to 15 weeks in the control group) and enhanced hemodynamics, manifested as lower mean arterial pressure (MAP) reduction and heart rates post-block. Notably, the basilic vein diameter post-block was greater in the BPB group, suggesting improved conditions for AVF creation and maturation. Additionally, instances of emergence agitation were reduced from 66% in the control group to 20% in the BPB group, indicating a smoother recovery phase. Pain scores at varying time intervals after surgery were also significantly lower for those receiving BPB, demonstrating effective analgesia.
Conclusion and Future Directions
This trial underscores the role of regional anesthesia as a feasible approach in pediatric patients undergoing AVF surgery. By fostering vasodilation and facilitating adequate blood flow, BPB not only reduces the chances of AVF failure but also enhances the overall surgical experience through better hemodynamic management and reduced perioperative discomfort. The findings advocate for the integration of ultrasound-guided regional anesthesia techniques into the standard anesthetic regimen for pediatric patients with ESRD, aiming for improved surgical outcomes and patient comfort. Future research is encouraged to further explore long-term effects and validate these findings across larger cohorts.
Key Points
– Pediatric renal disease complicates anesthetic management due to increased morbidity and mortality, necessitating effective perioperative pain control strategies, such as pediatric regional anesthesia.
– The study focuses on ultrasound-guided supraclavicular brachial plexus block (BPB) as an adjunct to sevoflurane anesthesia for improving outcomes during brachiobasilic arteriovenous fistula (AVF) surgeries in children with end-stage renal disease (ESRD).
– In a randomized controlled trial involving 60 pediatric ESRD patients, two groups underwent either general anesthesia with BPB or with saline injection, assessing AVF maturation time, hemodynamic stability, emergence agitation, and postoperative analgesia.
– BPB was associated with significantly faster AVF maturation (12 weeks vs. 15 weeks in the control group), better hemodynamic parameters, and a larger basilic vein diameter, which are vital for successful AVF creation.
– Emergence agitation was markedly reduced in the BPB group (20%) compared to the control group (66%), indicating improved recovery experiences, along with significantly lower pain scores at various postoperative intervals.
– The findings support the use of regional anesthesia techniques like BPB in pediatric patients with ESRD undergoing AVF surgery, emphasizing potential benefits in surgical outcomes and patient comfort while highlighting the need for further research to confirm these results in larger populations.
Reference –
S. Elsawy et al. (2025). Ultrasound-Guided Supraclavicular Brachial Plexus Block As An Additive To Sevoflurane Anesthesia In Pediatrics Undergoing Brachiobasilic Arteriovenous Fistula Operation: Randomized Controlled Clinical Trial. *BMC Anesthesiology*, 25. https://doi.org/10.1186/s12871-025-03091-1.