Faster lymph flow in the legs is linked to a better response to diuretics in acute heart failure patients
Powered by WPeMatico
Powered by WPeMatico

Netherlands: A new study has shown that eye movement desensitization and reprocessing (EMDR) therapy led to significant reductions in personality disorder (PD) symptoms, with nearly half of the participants achieving diagnostic remission, highlighting its potential as an effective treatment option.
Powered by WPeMatico
According to a new study published in JAMA, in unselected patients with acute myocardial infarction, routine Helicobacter pylori screening did not significantly lower the risk of upper gastrointestinal (GI) bleeding. The study was published by Robin H. and colleagues. Upper GI bleeding is an established complication following myocardial infarction and frequently attributed to the anticoagulant or antiplatelet therapy.
The objective of this study was to identify if routine H. pylori screening during hospitalization for myocardial infarction could decrease upper gastrointestinal bleeding and enhance clinical outcomes versus usual care. This multicenter, open-label, cluster randomized, crossover trial was performed at 35 Swedish hospitals, organized in 18 clusters. The trial lasted from November 17, 2021, to January 17, 2024, with follow-up on January 17, 2025. The hospital clusters were each subjected to two one-year periods: one with usual H. pylori screening of all patients admitted with myocardial infarction, and one with standard care, with a two-month washout interval between them.
During the periods of screening, patients received H. pylori testing with the urea breath test alongside routine post–myocardial infarction care. Information was obtained by means of a national clinical registry combined with Swedish health data registries. The end point was upper gastrointestinal bleeding and was analyzed with a negative binomial model in an intention-to-treat population.
Results
18,466 patients (median 71 years, interquartile range 61–79 years) were enrolled, including 13,138 males (71%). 9245 of the patients had been hospitalized during the screening periods for H. pylori, and 9221 during nonscreening (usual care) periods.
At hospital admission, 2284 patients in the screening group and 2275 in the nonscreening group (both 24.7%) used proton pump inhibitors (PPIs).
Among 6480 patients (70%) screened during the screening periods, 1532 (23.6%) were positive for infection. After a median of 1.9 years’ follow-up, 299 patients in the screening group had upper gastrointestinal bleeding, compared with 336 in the control group.
The event rate was 16.8 events per 1000 person-years in the screening group and 19.2 events per 1000 person-years in the usual care group, yielding a rate ratio (RR) of 0.90 (95% CI, 0.77–1.05; P = 0.18).
Subgroup analysis found the effect of screening to differ depending on admission anemia status.
The rate ratio was 0.98 (95% CI, 0.80–1.21) among nonanemic patients, with no difference.
For those with mild anemia, RR was 0.64 (95% CI, 0.42–0.98), and for those with moderate to severe anemia, RR was 0.44 (95% CI, 0.23–0.87), suggesting benefit of screening among these subgroups (P for interaction = 0.03).
The study concluded that among hospitalized patients with acute myocardial infarction, routine Helicobacter pylori screening had no significant protective effect against upper gastrointestinal bleeding in comparison with standard care. Future studies might consider selective screening of high-risk subgroups to maximize gastrointestinal protection during cardiac therapy.
Reference:
Hofmann R, James S, Sundqvist MO, et al. Helicobacter pylori Screening After Acute Myocardial Infarction: The Cluster Randomized Crossover HELP-MI SWEDEHEART Trial. JAMA. 2025;334(13):1160–1169. doi:10.1001/jama.2025.15047
Powered by WPeMatico
The FDA has approved oral remibrutinib as the first BTK inhibitor for chronic spontaneous urticaria in adults not responding to antihistamines.
Rhapsido is a pill taken twice daily and does not require injections or lab monitoring. It is the first FDA-approved Bruton’s tyrosine kinase inhibitor (BTKi) for CSU. Rhapsido helps to inhibit the release of histamine and other proinflammatory mediators by targeting BTK, offering a unique approach to CSU treatment.
“CSU is a serious disease that can cause debilitating symptoms and unpredictable flares. It’s difficult to diagnose and manage,” said Mark Lebwohl, MD, Dean for Clinical Therapeutics at the Icahn School of Medicine at Mount Sinai and member of the steering committee for the remibrutinib REMIX Phase III clinical trial program. “Remibrutinib represents a new way of treating CSU. By blocking the activity of BTK, remibrutinib stops a key pathway of the immune response in CSU. This is an exciting new option that has the potential to help a broad range of patients get fast relief.”
CSU is a mast cell-driven condition thought to be caused by immune dysregulation. In people with CSU, the immune system can become activated through allergic (IgE) or autoimmune (IgG) pathways. This causes certain immune cells-mast cells and basophils-to activate the BTK protein. While not fully understood, it is believed that once activated, BTK leads to the release of histamine and other proinflammatory mediators that may cause the red, swollen, and itchy hives commonly seen in CSU.
CSU symptoms are unpredictable, recurring for six weeks or more without an identified cause. Diagnosis can take up to 24 months.8 Many CSU patients say their symptoms negatively impact their sleep, work, and mental health.9,10,11 Antihistamines are the first-line treatment, but over half of patients still have symptoms, even at higher doses. Injectable treatments exist for those who don’t respond to antihistamines, yet fewer than 20% of eligible patients receive them.
“The approval of remibrutinib is an important development in CSU care. It quickly reduces symptoms, offering patients control of the hives and itching that they experience on a daily basis,” said Giselle Mosnaim, MD, MS, an Allergist and Immunologist from Endeavor Health, Clinical Associate Professor at the University of Chicago Pritzker School of Medicine and REMIX trial investigator. “This is significant because it expands beyond existing injectable treatments and gives patients an oral option that can easily be incorporated into their daily lives.”
“Many CSU patients feel misunderstood and settle for treatments that don’t fully meet their needs,” said Lynda Mitchell, CEO of Allergy & Asthma Network. “We support new treatment options that empower patients to choose what works best for them. This convenient new oral therapy offers a promising new way to manage CSU and potentially improve daily life for those living with this challenging condition.”
The FDA approval of Rhapsido in CSU is based on results from the Phase III REMIX-1 (NCT05030311) and REMIX-2 (NCT05032157) clinical trials in patients who remained symptomatic on second-generation H1 antihistamines. Rhapsido demonstrated superiority in change from baseline versus placebo in itch (ISS7), hives (HSS7), and weekly urticaria activity (UAS7) at Week 12. Significantly more patients treated with Rhapsido versus placebo achieved well-controlled disease (UAS7≤6) as early as Week 2 and at Week 12, and about one-third of patients achieved complete absence of itch and hives at Week 12. Rhapsido has a demonstrated safety profile that requires no lab monitoring.13 The most common adverse events (incidence ≥3%) were nasal congestion, sore throat, and runny nose (nasopharyngitis), bleeding, headache, nausea, and abdominal pain.
Novartis has completed regulatory submissions for Rhapsido for the treatment of CSU across many countries, including in the European Union, Japan, and China, with priority review granted in China.
“This approval of Rhapsido as the first and only BTK inhibitor in CSU is an important milestone in our journey to reshape care for overlooked immune-related conditions and offer more patients the potential to find fast relief,” said Victor Bultó, President, US, Novartis. “Building on our legacy in advancing the treatment of allergic, dermatologic, and rheumatologic conditions, we are deeply committed to further investing in innovative, patient-focused therapies across immunology.”
Discovered and developed by Novartis to target the BTK pathway as a driver of inflammation, remibrutinib is being investigated in ongoing clinical trials across a variety of immune-related conditions, including chronic inducible urticaria (CIndU), hidradenitis suppurativa (HS), and food allergy.
Powered by WPeMatico
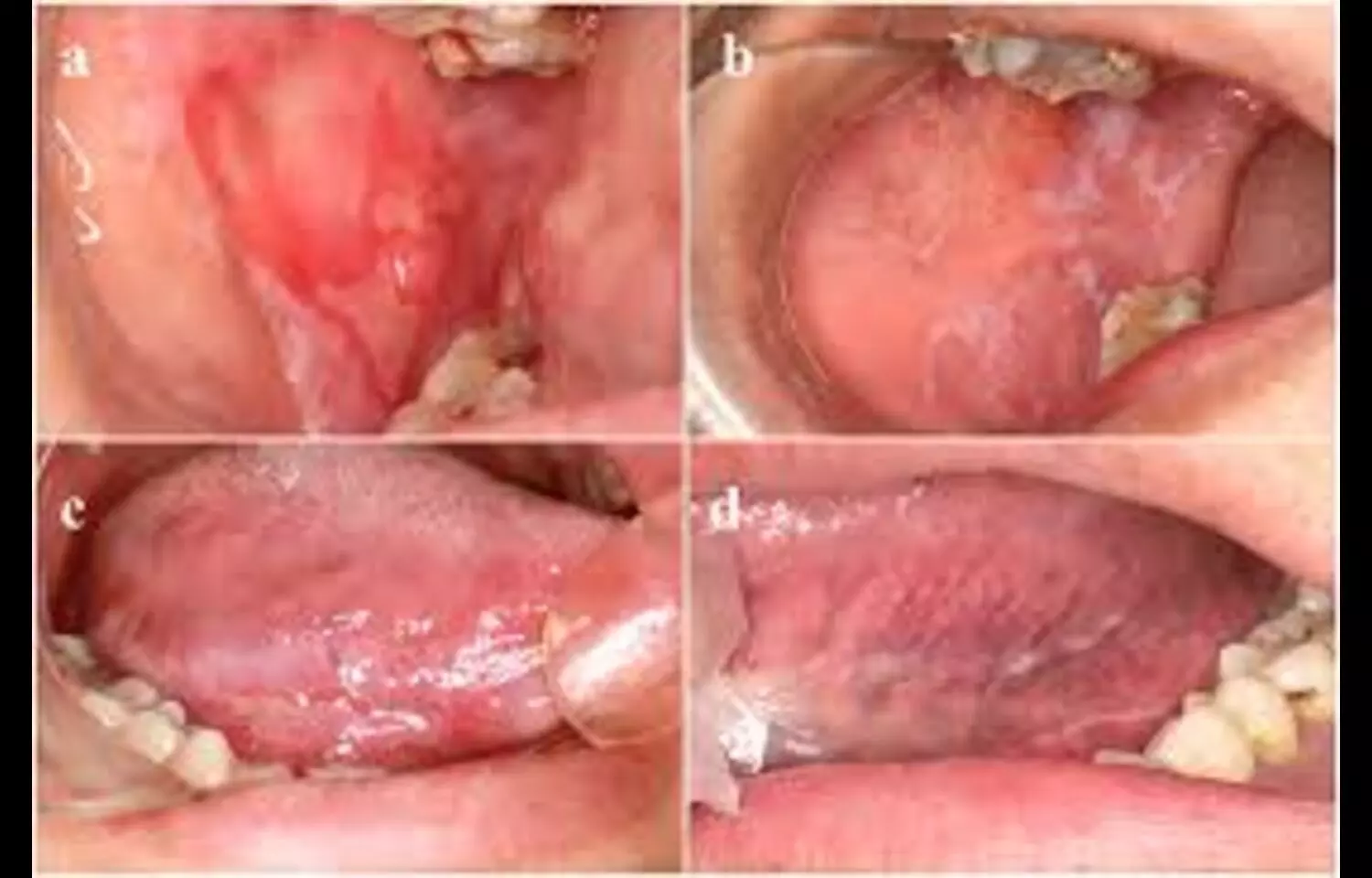
In a new study, curcumin lozenges demonstrated comparable pain relief to triamcinolone acetonide 0.1% paste after one and two weeks, while also reducing lesion size, pain, and burning sensation. The findings, published in Cureus by Harakh Chand Baranwal, Neelam Mittal, and colleagues, suggest that curcumin may serve as a safe and effective therapy for oral lichen planus, offering an alternative to topical corticosteroids with minimal side effects. Oral lichen planus is a chronic inflammatory condition that can cause significant discomfort, and identifying treatments that provide relief while minimizing systemic or local adverse effects remains a key focus of clinical research.
The pilot clinical study assessed adult patients with erosive oral lichen planus who were treated with either curcumin lozenges or 0.1% triamcinolone acetonide paste over a two-week period. Both interventions were found to alleviate pain and reduce burning sensations, but curcumin additionally showed a favorable impact on lesion size and mucosal healing. These results highlight the anti-inflammatory and antioxidant properties of curcumin as the likely mechanisms behind its therapeutic effect. Moreover, curcumin’s natural profile and low side effect burden make it particularly appealing for long-term management of a chronic oral condition that often requires repeated interventions.
The authors conclude that curcumin lozenges could represent a viable alternative or adjunct to corticosteroid therapy in managing erosive oral lichen planus. Given the chronic nature of the disease and the potential side effects associated with long-term corticosteroid use, curcumin offers a promising option that is both effective and well-tolerated. Further large-scale studies are warranted to confirm these findings, optimize dosing, and explore long-term outcomes. This study supports the growing interest in natural and complementary therapies in oral medicine, emphasizing patient safety and quality of life alongside clinical efficacy.
Keywords: oral lichen planus, curcumin lozenges, triamcinolone acetonide, pain relief, lesion healing, anti-inflammatory therapy, pilot clinical study, Cureus, Harakh Chand Baranwal, Neelam Mittal
Powered by WPeMatico

Diagnosis of Seronegative Rheumatoid Arthritis (SNRA) is based on clinical, radiological, and MRI findings with set criteria. High specificity is provided by plain X-rays in the differential diagnosis of rheumatic disorders.
In the study by C Rex et al of pelvis X-rays, they found that ischiopubic ramus enthesopathy was more common than sacroiliitis and they have coined that enthesopathy finding as “hanging drop sign” because of its characteristic appearance.
A total of 152 proven (92 Females, 60 Males) SNRA cases (based on ACR*/EULAR**criteria and ASAS***criteria) were studied for enthesopathy features in their plain X-ray of pelvis. In addition to sacroiliitis, calcification/ ossification of attachment of pelvic muscle and ligaments was documented. Hanging drop enthesopathy was noted in ischiopubic ramus in most of the cases. A cadaveric study was done on 10 specimens just to understand the corresponding anatomical origin of this enthesopathy.
The key findings of the study were:
• Of the 152 pelvic radiographs studied, 110 revealed radiological evidence of SNRA.
• Among them, 64 patients had hanging drop sign (58.18%), 25 patients had sacroiliitis (22.72%), and 21 patients had other features like hip arthritis, calcifications around greater trochanter, iliac crest, and acetabular region.
• This hanging drop sign can be single (n = 52) or multiple (n = 12) and can be unilateral (n = 14) or bilateral (n = 50).
• The cadaveric study revealed that the hanging drop sign corresponds to the adductor part of adductor magnus muscle and gracilis muscle origin in the ischiopubic ramus.
“We conclude that the incidence of ischiopubic ramus enthesopathy presenting as “hanging drop sign” is higher than the incidence of sacroiliitis. Hanging drop sign can be considered as a reliable complementing sign to identify SNRA using standard diagnostic criteria. However, large volume and larger follow-ups are required to confirm the prevalence in various subtypes of axial spondyloarthropathies” the author commented.
Further reading:
“Hanging Drop Sign” in pelvis X-ray
C. Rex et al
Indian Journal of Orthopaedics (2025) 59:644–649
https://doi.org/10.1007/s43465-025-01360-2
Powered by WPeMatico
A meta-trial of randomized clinical studies across six countries has found that inhaled heparin significantly reduced the need for intubation, overall mortality, and in-hospital death among hospitalized COVID-19 patients.
A widely available and affordable drug has been shown to be effective in treating seriously ill COVID-19 patients, according to a new international study led by researchers at the Australian National University (ANU) in collaboration with King’s College London.
The study analysed data from almost 500 patients hospitalised with COVID-19 across six countries. Patients who inhaled heparin were half as likely to require ventilation and had a significantly lower risk of dying compared with those receiving standard care.
Heparin, a drug traditionally injected to treat blood clots, was tested in this study in an inhaled form, targeting the lungs directly. As well as acting as an anticoagulant, heparin has anti-inflammatory and pan-antiviral properties. Earlier research results showed breathing and oxygen levels improved in COVID-19 patients after they inhaled a course of heparin.
The researchers believe the drug could also be useful in fighting other serious respiratory infections such as pneumonia.
Professor Clive Page, Emeritus Professor of Pharmacology at King’s who co-led the international study with ANU’s Professor van Haren, said: “Inhaled heparin is anti-viral, anti-inflammatory and anti-coagulant. There’s no other drug that has that unique combination. We know it’s only a matter of time until the next pandemic, and there are still COVID-19 patients who get very sick. This is a great weapon to have up our sleeve.”
While the findings highlight the potential of inhaled heparin, further development is required before the treatment can be routinely adopted. The researchers believe the drug could also be useful in fighting other serious respiratory infections such as pneumonia, which can be caused by a range of viruses and bacteria.
According to Professor van Haren, the drug would also be helpful for those with a compromised immune system, such as cancer patients, when they experience a respiratory infection.
Professor Frank van Haren, lead author, ANU and Director of the Intensive Care Unit at the St George Hospital in Sydney, said: “It doesn’t matter what kind of respiratory infection the patient is dealing with, the drug – when inhaled – will stop it from infecting the patient and from damaging the lungs. We’re aiming to conduct another trial in Europe to confirm its effectiveness in fighting other common respiratory infections such as influenza and RSV. And because it’s inexpensive, it’s much more accessible for those from low-income countries”
The researchers are now also developing an improved formulation of heparin, specifically designed to be given by inhalation.
Reference:
van Haren, Frank M.P. et al., Efficacy of inhaled nebulised unfractionated heparin to prevent intubation or death in hospitalised patients with COVID-19: an investigator-initiated international meta-trial of randomised clinical studies, EClinicalMedicine.
Powered by WPeMatico

Research published in Cancer Discovery suggests that high consumption of the artificial sweetener sucralose may reduce the effectiveness of immune checkpoint inhibitors (ICIs) in cancer patients. The research revealed that sucralose disrupted the balance of the gut microbiota, inhibited T cell function and metabolism, and eventually dampened the efficacy of immune checkpoint inhibitors (ICIs), i.e., anti-PD-1 based treatments. The study was published by Kristin M. and fellow researchers.
The study probed the way diet affects gut microbiota and downstream immune responses in preclinical models of cancer and advanced cancer patients treated with ICI. Although the gut microbiota is known to influence cancer immunity, the particular effect of non-nutritive sweeteners was uncertain. In this research, direct evidence was given that sucralose intake altered microbial composition and decreased the pool of microbiota-accessible arginine, an amino acid necessary for T cell function.
Key Findings
1 shared sweetener (sucralose) profoundly changed gut microbiota composition.
T cell metabolism and function were limited under sucralose treatment.
Anti-PD-1 immunotherapy efficacy was decreased in preclinical cancer models and patients with advanced cancer.
Arginine levels available to the microbiota decreased following sucralose consumption.
Amino acid supplementation or fecal microbiota transfer (FMT) from responder mice fully restored T cell function and immunotherapy results.
The researchers isolated the biological process underlying sucralose’s adverse effect. Sucralose changed microbial makeup within the gut, which then decreased the microbial pool of available arginine. Arginine is instrumental in T cell activation and metabolism. Without adequate arginine, T cells lost their functional abilities, restricting their capacity to develop effective immune responses against cancers. Restoring arginine levels—either by supplementation or microbiota transfer—restored normal T cell function and response to immunotherapy.
This research determined that sucralose intake destabilizes microbiota of the gut, decreases microbiota-accessible arginine, disrupts T cell metabolism, and eliminates response to anti-PD-1 immunotherapy. Notably, resupplementation of amino acids or microbiota transfer from responder subjects negated the adverse effects. These findings emphasize the necessity of dietary consideration in cancer therapy, as an ubiquitous artificial sweetener could perilously undermine lifesaving immunotherapies.
Reference:
Powered by WPeMatico

New Delhi: In the wake of the tragic child deaths in Chhindwara, Madhya Pradesh, allegedly linked to contaminated cough syrups, the Central Drugs Standard Control Organisation (CDSCO) has issued a strong directive to all State and Union Territory Drug Controllers, calling for strict enforcement of the Drugs Rules, 1945, with specific emphasis on the mandatory testing of raw materials and finished pharmaceutical products.
In an official communication, the Drugs Controller General of India (DCGI), Dr Rajeev Singh Raghuvanshi, emphasized that manufacturers must test every batch of raw materials, including excipients and active pharmaceutical ingredients (APIs)—as well as the finished formulations before their release to the market.
The directive comes in the backdrop of inspection findings that exposed serious lapses by certain manufacturers, who failed to conduct the required batch-wise testing of raw materials and finished formulations, in violation of Rules 74(c) and 78(c)(ii) of the Drugs Rules, 1945.
It is noted that during the inspections carried out at the manufacturing facilities and in the investigations of the drugs declared as Not of Standard Quality, it was observed in the reports that the manufacturers are not carrying out testing of each batch of the excipients/inactive and active pharmaceutical ingredients for verification of compliance with the prescribed standards before using them in the manufacture of formulations and also in the finished products.
In addition to the above, the Drugs Controller General of India (DCGI) stated,
“It is to mention that as per the Drugs Rules, including rule 74 (c) and rule 78 (c) (ii), the licensee shall, either in his own laboratory or in any laboratory approved by the licensing authority test each batch or lot of the raw material used by him for the manufacture of his product and also each batch of the final product and shall maintain records or registers showing the particulars in respect of such tests as specified in Schedule U.”
Accordingly, the Drugs Controller General of India (DCGI) directed,
“All State and UT Drug Controllers be instructed to monitor compliance during inspections, issue sensitization circulars to manufacturers, and ensure that companies maintain robust vendor qualification systems. Manufacturers have also been told to procure raw materials only from approved and reliable suppliers.”
Powered by WPeMatico
Powered by WPeMatico
