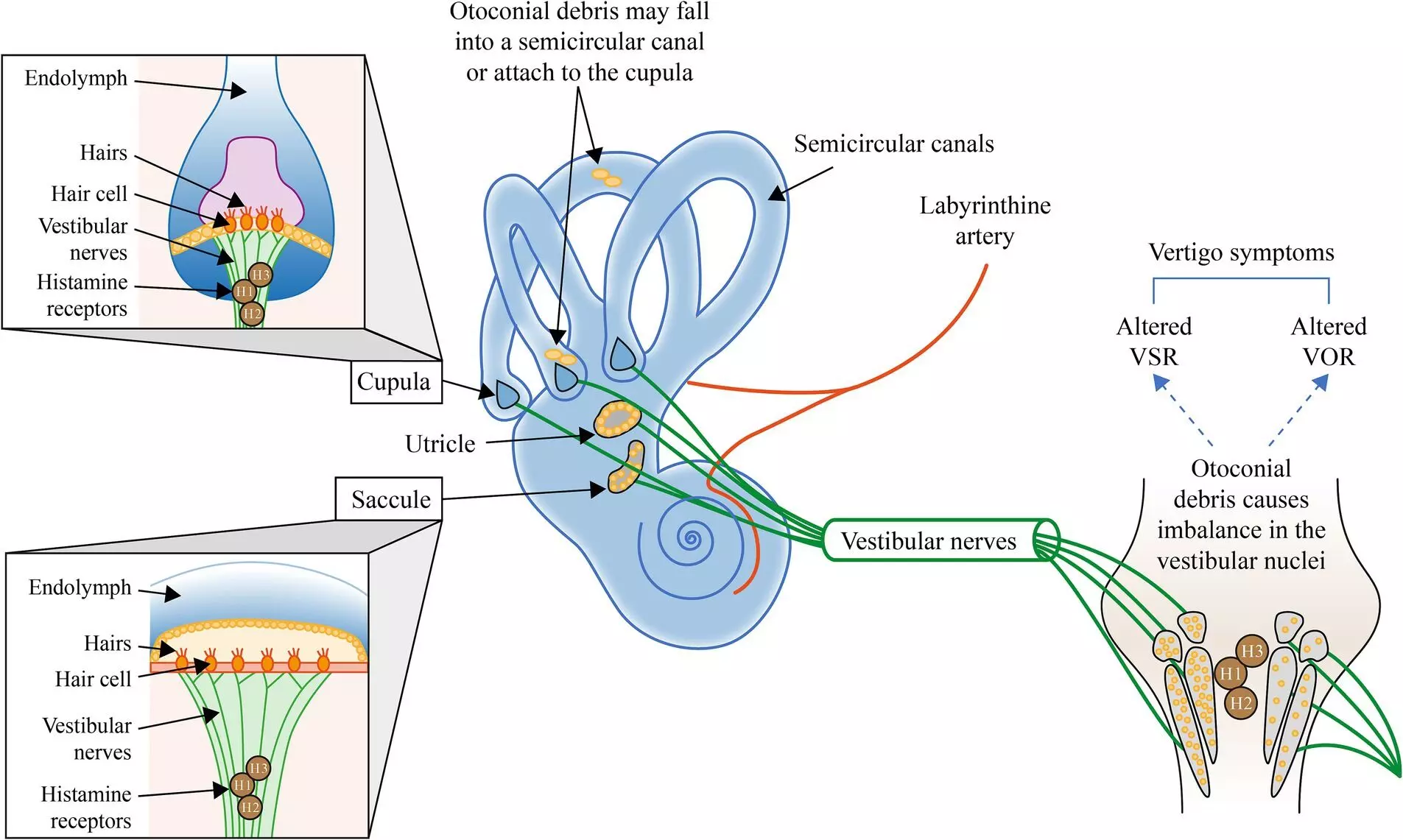
Recently published research assessed copeptin levels during the entire perioperative period of open adult cardiac surgeries involving cardiopulmonary bypass (CPB) in a prospective cohort study at a tertiary care hospital. The study included 61 patients, but ultimately analyzed data from 57 after exclusions for specific surgical conditions. Patients were sorted into subgroups based on their preoperative copeptin levels—either above or below 10 pmol/L, the established reference threshold.
Importance of Copeptin
Copeptin, a precursor of arginine vasopressin (AVP), has been recognized as a prognostic marker for various cardiovascular conditions, including heart failure and myocardial infarction. Several earlier studies indicated that copeptin levels could increase significantly during CPB and potentially correlate with adverse surgical outcomes. This investigation aimed to fill the gap in knowledge surrounding the dynamic changes in copeptin levels throughout the perioperative course. Preoperative levels of copeptin were measured a day before surgery, with subsequent samples collected at several critical time points: after sternotomy, at the commencement of CPB, during the procedure every 30 minutes, post cross-clamping, following CPB cessation, and at several intervals up to four days post-surgery. Copeptin was quantified in a laboratory using a well-validated method, ensuring accuracy in data.
Results Analysis
In analyzing the results, a significant rise in copeptin levels was observed following sternotomy and continuation during CPB, peaking around 60 minutes into bypass. Beyond this, levels plateaued rather than further increasing, indicating a maximum hormone production phase during CPB. Following the termination of CPB, copeptin levels began to decline, reaching near baseline values by the first postoperative day but remaining elevated up to day four. Subgroup analysis indicated individuals with higher preoperative copeptin had significantly elevated levels postoperatively, suggesting that initial copeptin levels may relate to responses during and after surgery.
Statistical Findings
The assessments confirmed substantial statistical differences across multiple perioperative time points concerning copeptin release, with a notable peak corresponding to critical perioperative events. However, while copeptin acts as a reliable marker for hemodynamic stress during CPB, exact physiological mechanisms for its release in this context remain unclear.
Study Limitations
Study limitations noted the underrepresentation of women and the relatively small sample size, potentially affecting the generalizability of findings. The variations in patient numbers over time during CPB also posed challenges for precise subgroup analyses.
Conclusion and Future Directions
In conclusion, copeptin levels were distinctly characterized through various perioperative phases of cardiac surgery. The results emphasize the role of copeptin as an active biomarker that reflects physiological changes occurring during cardiac surgery with CPB. The study advocates for continued research to explore copeptin’s prognostic potential further and enhance risk assessment models in cardiac surgical procedures.
Key Points
– A prospective cohort study was conducted involving 61 patients undergoing open adult cardiac surgeries with cardiopulmonary bypass (CPB), with final analysis based on 57 patients after excluding specific cases; patients were categorized based on preoperative copeptin levels (above or below 10 pmol/L).
– Copeptin, a precursor to arginine vasopressin (AVP), serves as a prognostic marker for cardiovascular conditions such as heart failure and myocardial infarction, with prior studies indicating a significant rise during CPB linked to adverse surgical outcomes.
– Preoperative copeptin levels were measured one day before surgery, followed by multiple sampling points during the perioperative period, including after sternotomy, at the commencement of CPB, and several intervals up to four days post-surgery, with accurate quantification using validated laboratory methods.
– The results revealed a significant increase in copeptin levels after sternotomy, with a peak around 60 minutes into CPB, which plateaued during the procedure and began to decline after CPB cessation, though remained elevated up to four days post-surgery; higher preoperative copeptin levels correlated with elevated levels postoperatively.
– Statistical analysis demonstrated significant differences in copeptin release across various perioperative time points, peaking during critical events, yet the exact physiological mechanisms for copeptin release during CPB remain poorly understood.
– Limitations included a small sample size and underrepresentation of women, which may impact the generalizability of the findings; variability in patient numbers during CPB also complicated subgroup analyses, underscoring the need for further research into copeptin’s prognostic capabilities in cardiac surgery risk assessments.
Reference –
Selma Samuelsson et al. (2025). Copeptin Levels During Cardiac Surgery.. *Journal Of Cardiothoracic And Vascular Anesthesia*. https://doi.org/10.1053/j.jvca.2025.02.014.