Long working hours may alter brain structure, preliminary findings suggest
Powered by WPeMatico
Powered by WPeMatico
Powered by WPeMatico
Powered by WPeMatico
Powered by WPeMatico

Rajouri: The Government Medical College (GMC) and Hospital in Rajouri has announced that it will withhold the salaries of several staff members who were found absent from duty during the recent armed conflict between India and Pakistan, an official said on Tuesday. The period of absence shall be treated as unauthorized absence for these employees.
The punished workers include faculty, consultants, senior residents, junior residents, staff nurses, paramedical staff, technical staff, ministerial staff, multi-tasking staff, interns, and many others who fled the town on May 7, the order read.
“Furthermore, the said period shall be treated as unauthorized absence, and appropriate entries shall be made in their service records/service books in accordance with the applicable rules and regulations,” Principal, GMC Rajouri Prof (Dr) A S Bhatia said in the order, news agency PTI reported.
Also Read:Delhi Hospitals uphold Emergency Preparedness Despite India-Pakistan Ceasefire
The facility serves as a key medical institution for the entire Pir Panjal region spread across border districts of Rajouri and Poonch – both worst-hit in the artillery and mortar shelling by Pakistan.
At least 20 of the total 28 deaths in the conflict in Jammu and Kashmir were reported from the two districts, with over 50 others injured.
The dead included Rajouri Additional District Development Commissioner Raj Kumar Thappa, who lost his life when a shell hit his residential compound in Rajouri town on May 10.
Medical Dialogues had earlier reported that the Government Medical College Hospital (GMCH) Jammu has officially withdrawn a circular it issued on April 25, 2025, which had instructed hospital staff to remain on high alert due to reported cross-border tensions in the Union Territory of Jammu & Kashmir.
The earlier circular, issued by the Medical Superintendent of GMCH Jammu, had directed all staff to remain on high alert and ensure complete preparedness to meet any exigencies that may arise at any time. These measures included keeping essential medicines and critical equipment ready, limiting unnecessary leave, and ensuring uninterrupted patient care.
Powered by WPeMatico

Hyderabad: CuraTeQ Biologics s.r.o., a wholly owned step-down subsidiary of Aurobindo Pharma Ltd, has obtained
marketing authorisation from UK’s Medicines and Healthcare products Regulatory Agency (MHRA) for
Zefylti, its filgrastim biosimilar version.
Earlier this year, in February, the European Commission (EC) has granted marketing authorization in the European Union (EU) for Zefylti, to CuraTeQ Biologics s.r.o.., as previously reported by Medical Dialogues.
This is CuraTeQ’s second
biosimilar to be approved by MHRA after the approval of Bevqolva in December 2024.
Zefylti is intended for the treatment of neutropenia and the mobilisation of peripheral progenitor cells (PBPCs).
Read also: Aurobindo Pharma arm gets marketing authorisation for Zefylti in EU
CuraTeQ Biologics Private Limited, a wholly owned subsidiary of Aurobindo Pharma Limited, is
a global biopharmaceutical company headquartered in Hyderabad, India. It is focused on developing biosimilars for the treatment of various
cancers and autoimmune diseases. CuraTeQ’s pipeline consists of fourteen biosimilars, primarily
targeting the immunology and oncology segments. It has end-to-end capabilities in producing a full
range of products from bulk drug substance to fill-finish and packaged drug products.
Powered by WPeMatico
Cardiovascular diseases (CVD) account for 24.8% of all deaths in India, with an age-standardized death rate of 272 per 100,000-higher than the global average. (1) Indians develop CVD nearly a decade earlier than Western populations, with 62% of deaths occurring prematurely. Key cardiometabolic risk factors-hypertension, diabetes, and obesity-have driven a 2.3-fold rise in ischemic heart disease and stroke. (2)
CVD complications are also evident in acute settings: 20% of adults hospitalized with pneumococcal pneumonia experience heart failure, arrhythmia, or myocardial infarction. (3) Nearly 12% of patients developed heart failure within a decade of hospitalization, highlighting the prolonged cardiovascular impact of systemic infections such as pneumonia. (4)
Cardiopulmonary Overlap and Risk of Respiratory Infections in CVD
Cardiovascular and respiratory diseases frequently coexist due to shared risk factors such as smoking and systemic inflammation.(5) Symptom overlap is common-cardiac conditions like heart failure may present with respiratory features (e.g., cardiac asthma), while chronic lung disease increases right ventricular strain, predisposing to CHF. (6)
The risk intensifies with respiratory infections. Patients with cardiovascular disease—including CHF, valvular disease, and congenital heart disease—face a 3.3-fold higher risk of community-acquired pneumonia (CAP) and a 9.9-fold higher risk of invasive pneumococcal disease (IPD). (7) During IPD, Streptococcus pneumoniae translocate across the vascular endothelium and invades the myocardium, directly killing cardiomyocytes and immune cells. (8) This results in acute myocardial injury and can lead to long-term cardiac dysfunction, compounding the cardiovascular burden in affected patients. (9)
Clinical trials such as PARADIGM-HF and PARAGON-HF have demonstrated a significant incidence of pneumonia among heart failure patients. Pneumonia occurred in 6.3% (29 per 1,000 patient-years) of PARADIGM-HF patients and 10.6% (39 per 1,000 patient-years) of PARAGON-HF participants. The onset of pneumonia in these patients substantially increased the risk of adverse outcomes. (10)
Importance of Preventing Pneumococcal Disease in Cardiovascular Disease Patients
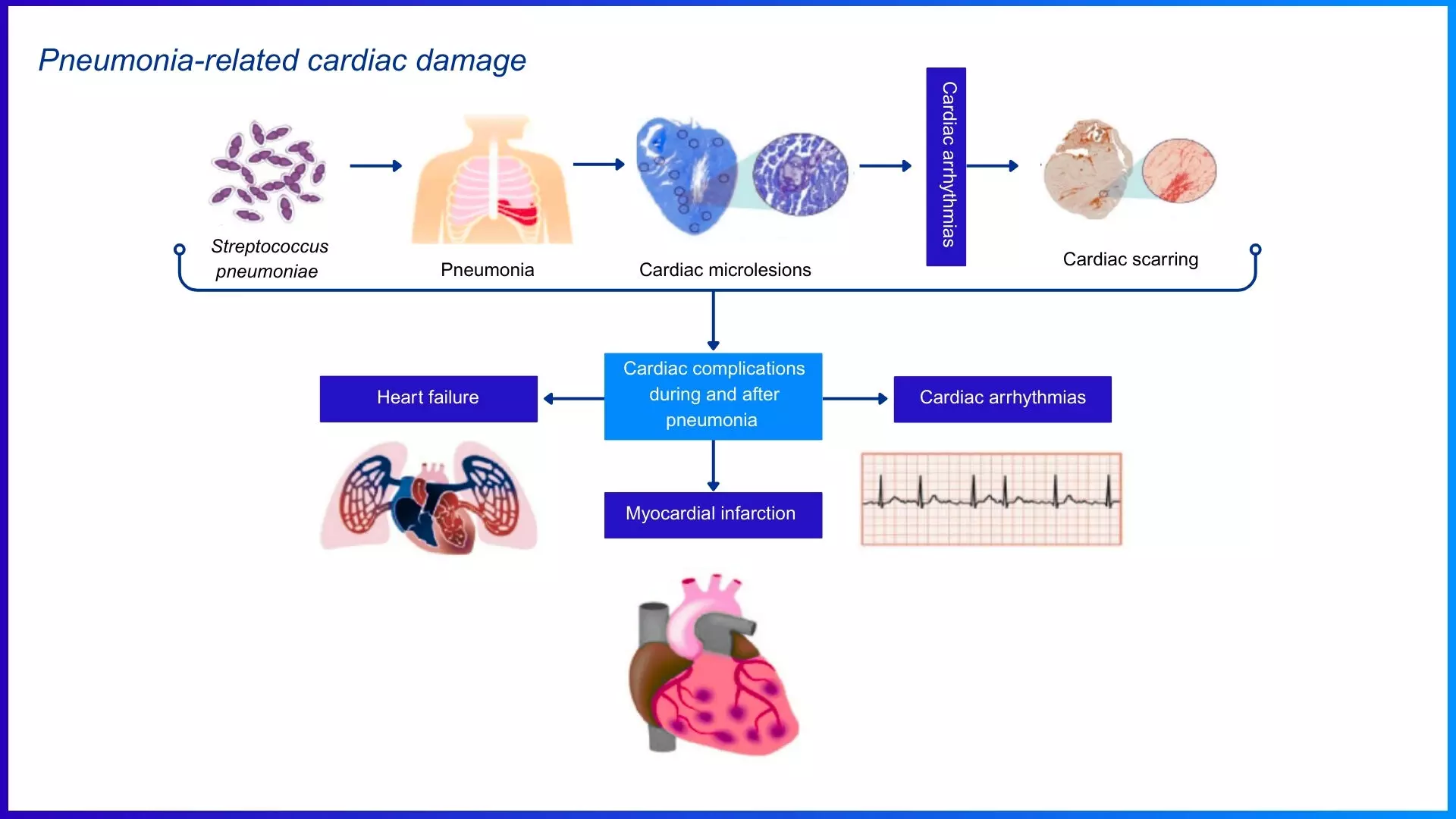
Figure: Streptococcus pneumoniae induces cardiovascular complications via multiple pathophysiological mechanisms. Adapted from Restrepo MI, Reyes LF. Respirology. 2018;23(3):250-259.
Preventing pneumococcal disease is vital for CVD patients, particularly those with heart failure and CAD, as they are more vulnerable to bacterial infections like pneumonia and other respiratory illnesses. These infections can trigger cardiac decompensation, increasing hospitalizations and mortality. Vaccination against pneumococcus lowers the risk of respiratory infections, heart failure exacerbations, and cardiovascular events. Given weakened immunity and constant exposure to airborne pathogens, vaccination helps stabilize heart function, reduce inflammation, and ease healthcare burdens. (11)
Pneumococcal Vaccination in Cardiovascular Diseases – When to Consider?
Table 1 provides adult vaccine recommendations for heart failure and related high-risk conditions per the API(Association of Physicians of India) 2024 guidelines.
Table 1: Vaccination Recommendations for High-Risk Cardiac and Chronic Conditions
|
High Risk Group |
Recommended Vaccines |
|
Heart Failure |
Pneumococcal (PCV + PPSV23), Influenza (annual), COVID-19 (annual), Zoster (≥50 yrs) |
|
Coronary Artery Disease |
Pneumococcal, Influenza, COVID-19 |
|
Valvular / Congenital HD |
Pneumococcal, Influenza, COVID-19 |
|
Post-Cardiac Surgery |
Pneumococcal, Influenza, COVID-19, Hepatitis B (if high exposure risk) |
|
CKD / Dialysis |
Pneumococcal, Hepatitis B, Influenza, COVID-19, Tdap |
|
Chronic Liver Disease |
Hepatitis A, Hepatitis B, Pneumococcal, Influenza, COVID-19 |
|
T2DM |
Pneumococcal, Influenza, Hepatitis B, COVID-19 |
|
Immunocompromised States (e.g. HIV, cancer) |
Pneumococcal, Influenza, Hepatitis A/B, COVID-19, HPV (if age-eligible) |
|
Age ≥50 |
Pneumococcal, Influenza, COVID-19, Zoster, Tdap |
Vaccination should be considered a priority for individuals with CVD, as they are inherently immunocompromised due to circulatory impairment and elevated inflammatory cytokines. Advancing age (55+), diabetes, and hypertension further increase infection risk and worsen CAD. Those with congenital or valvular heart disease and post-cardiac surgery patients also face high infection risks and must adhere to vaccination protocols, particularly for pneumococcal vaccine. (12)
Pneumococcal conjugate vaccine (PCV13) should be administered first, followed by the pneumococcal polysaccharide vaccine (PPSV23) after eight weeks. (13)
Vaccination in Cardiovascular Diseases – View of Scientific Authorities
Table 2 lists vaccines with Class recommendations for adults with cardiovascular and related chronic conditions. (13)
Table 2: Approved Class I Vaccine Recommendations in High-Risk Adults
|
Vaccine |
Class I Recommendations |
|
Influenza |
CVD, T2DM, lung disease, age ≥50 years, immunocompromised |
|
Pneumococcal |
CVD, age ≥50 years, T2DM, CKD, chronic lung disease |
|
Tdap / Td |
All adults every 10 years pregnancy |
|
Hepatitis B |
T2DM, CKD, chronic lung disease, high-risk behavior |
|
Herpes Zoster |
Age ≥50 years, immunocompromised, chronic heart and lung disease |
|
COVID-19 |
Annually, all with chronic comorbidities |
Leading health organizations strongly recommend pneumococcal and influenza vaccination for patients with chronic cardiovascular disease. The Centers for Disease Control and Prevention/Advisory Committee on Immunization Practices (CDC/ACIP) advises pneumococcal vaccination for all adults over 50 and high-risk cardiovascular patients, alongside annual influenza vaccination for those with chronic heart and lung diseases. (14) Table 3 summarizes key vaccination recommendations from major global and national health authorities.
Table 3: Pneumococcal Vaccination Recommendations by Leading Health Organizations
|
Guideline/Organization |
Vaccine Recommendation |
|
Indian Consensus Guidelines on Adult Immunization by the Association of Physicians of India [API, 2024] (13) |
Recommends pneumococcal vaccines for adults aged 18-49 years with underlying conditions (chronic heart, chronic lung, diabetes, liver, kidney disease) and all adults aged 50+ years. |
|
European Society of Cardiology [ESC, 2021] (15) |
Suggest pneumococcal vaccination as part of heart failure management. |
|
World Health Organization [WHO,2021] (16) |
Advises pneumococcal vaccine for older adults. |
|
American Heart Association [AHA, 2013] (15) |
Suggest pneumococcal vaccination as part of heart failure management. |
|
Heart Failure Society of America [HFSA, 2010] (11) |
Recommends pneumococcal vaccine for all heart failure patients. |
Additional recommended vaccines include Hepatitis B, which supports liver and overall health, and the shingles (herpes zoster) vaccine, as heart failure patients are prone to outbreaks due to stress, viral infections, and immune dysfunction.(12)
Effectiveness of Pneumococcal Vaccination in Improving Cardiovascular Outcomes
Vaccination significantly reduces hospitalizations and cardiovascular complications. A meta-analysis of 15 studies (347,444 patients) showed that pneumococcal vaccination reduced all-cause mortality by 24% (HR: 0.76, 95% CI: 0.66–0.87, p< 0.001) and myocardial infarction (MI) by 27% (HR: 0.73, 95% CI: 0.56–0.96, p = 0.02). (17) Another meta-analysis of seven studies (163,756 participants) showed pneumococcal vaccination reduced all-cause mortality by 22% (HR: 0.78, 95% CI: 0.73–0.83, p < 0.001). These findings highlight pneumococcal vaccination as a crucial preventive strategy for individuals at high cardiovascular risk, particularly those aged ≥65 years. (18)
Overcoming Barriers to Vaccination in Cardiac Patients – Multi-stakeholder Approach
Vaccine adherence remains low due to concerns about side effects, physician hesitancy, and cost. While cost is a barrier, hospitalization expenses for preventable infections are far higher. To improve adherence, public health initiatives, patient education, and enhanced accessibility are key. Effective strategies include patient messaging and community education to emphasize vaccines as essential for reducing cardiovascular risk and improving long-term health. (19)
Take Home Message
References:
1. Jan, Bisma et al. “Cardiovascular Diseases Among Indian Older Adults: A Comprehensive Review.” Cardiovascular therapeutics vol. 2024 6894693. 25 Jun. 2024.
2. Kalra, Ankur et al. “The burgeoning cardiovascular disease epidemic in Indians – perspectives on contextual factors and potential solutions.” The Lancet regional health. Southeast Asia vol. 12 100156. 10 Feb. 2023, doi:10.1016/j.lansea.2023.100156
3. Musher, Daniel M et al. “The association between pneumococcal pneumonia and acute cardiac events.” Clinical infectious diseases : an official publication of the Infectious Diseases Society of America vol. 45,2 (2007): 158-65. doi:10.1086/518849
4. Eurich, Dean T et al. “Risk of heart failure after community acquired pneumonia: prospective controlled study with 10 years of follow-up.” BMJ (Clinical research ed.) vol. 356 j413. 13 Feb. 2017, doi:10.1136/bmj.j413
5. Carter, Paul et al. “Association of Cardiovascular Disease With Respiratory Disease.” Journal of the American College of Cardiology vol. 73,17 (2019): 2166-2177. doi:10.1016/j.jacc.2018.11.063
6. Buckner, Kern. “Cardiac asthma.” Immunology and allergy clinics of North America vol. 33,1 (2013): 35-44. doi:10.1016/j.iac.2012.10.012
7. Torres, Antoni et al. “Which individuals are at increased risk of pneumococcal disease and why? Impact of COPD, asthma, smoking, diabetes, and/or chronic heart disease on community-acquired pneumonia and invasive pneumococcal disease.” Thorax vol. 70,10 (2015): 984-9. doi:10.1136/thoraxjnl-2015-206780
8. Brown, Armand O et al. “Streptococcus pneumoniae translocates into the myocardium and forms unique microlesions that disrupt cardiac function.” PLoS pathogens vol. 10,9 e1004383. 18 Sep. 2014, doi:10.1371/journal.ppat.1004383
9. Shenoy, Anukul T et al. “Streptococcus pneumoniae in the heart subvert the host response through biofilm-mediated resident macrophage killing.” PLoS pathogens vol. 13,8 e1006582. 25 Aug. 2017, doi:10.1371/journal.ppat.1006582
10. Shen L, Jhund PS, Anand IS, et al. Incidence and Outcomes of Pneumonia in Patients With Heart Failure. J Am Coll Cardiol. 2021;77(16):1961-1973.
11. Bhatt AS, DeVore AD, Hernandez AF, Mentz RJ. Can Vaccinations Improve Heart Failure Outcomes?: Contemporary Data and Future Directions. JACC Heart Fail. 2017;5(3):194-203.
12. Centers for Disease Control and Prevention. “Vaccination Information for Adults with Heart Disease.” CDC, https://www.cdc.gov/vaccines/adults/rec-vac/heart-disease-home.html. Accessed 19 Feb. 2025.
13. Indian Consensus Guideline on Adult Immunization.EMVAC, June 2024, https://www.emvac.in/wp-content/uploads/2024/06/Adult-Vaccination-Booklet.pdf. Accessed 25 Feb. 2025.
14. U.S. Centers for Disease Control and Prevention.CDC Recommends Lowering the Age for Pneumococcal Vaccination from 65 to 50 Years Old. Accessed on 2nd January 2025 from https://www.cdc.gov/media/releases/2024/s1023-pneumococcal-vaccination.html
15. Jaiswal V, Ang SP, Lnu K, et al. Effect of Pneumococcal Vaccine on Mortality and Cardiovascular Outcomes: A Systematic Review and Meta-Analysis. J Clin Med. 2022;11(13):3799. Published 2022 Jun 30. doi:10.3390/jcm11133799
16. World Health Organization. (n.d.). Pneumonia. Immunization, Vaccines and Biologicals. Retrieved from https://www.who.int/
17. Jaiswal V, Ang SP, Lnu K, et al. Effect of Pneumococcal Vaccine on Mortality and Cardiovascular Outcomes: A Systematic Review and Meta-Analysis. J Clin Med. 2022;11(13):3799. Published 2022 Jun 30.
18. Marques Antunes M, Duarte GS, Brito D, et al. Pneumococcal vaccination in adults at very high risk or with established cardiovascular disease: systematic review and meta-analysis. Eur Heart J Qual Care Clin Outcomes. 2021;7(1):97-106.
19. García-Zamora S, Pulido L. Vaccines in cardiology, an underutilized strategy to reduce the residual cardiovascular risk. Arch Peru Cardiol Cir Cardiovasc. 2024;5(1):29-39. Published 2024 Mar 19.
Powered by WPeMatico

Mohali: In a bid to address the increasing shortage of medical professionals in government hospitals, Punjab Health and Family Welfare Minister Dr. Balbir Singh announced on Monday that the recruitment process for 1,000 doctors has already commenced.
Medical Dialogues had previously reported that in a major boost to the state’s healthcare infrastructure, the Punjab Government has decided to recruit 1,000 Medical Officers (MBBS). The decision was revealed during a meeting held between Principal Secretary Health and Family Welfare Kumar Rahul, Director Health Services Dr. Hitinder Kaur, Director PHSC Dr. Anil Goyal, and representatives of the Punjab Civil Medical Services Association (PCMSA).
Dr. Singh announced a surprise inspection of healthcare facilities in Dera Bassi, Lalru, and Zirakpur (Dhakoli). During his visit, he observed staffing shortages and inadequate infrastructure at some hospitals, which are currently struggling to meet the growing demand for healthcare services. The minister took the opportunity to interact with patients and their families, gaining first-hand insight into the challenges faced by the public in accessing healthcare services.
Also Read: Punjab set to recruit 1000 Medical Officers
According to Hindustan Times, “The Punjab government is committed to delivering quality healthcare services in government hospitals, which include free lab tests and medicines. Specialists in medicine, surgery, gynaecology and pediatrics are available in most sub-divisional hospitals and community health centres (CHCs),” he said, adding that eye, ENT and dermatology specialists would be assigned on a weekly basis at the facilities having these positions vacant.
The minister highlighted that the CHC in Lalru was well-equipped in terms of infrastructure, and plans were in place to recruit additional staff to enhance service delivery. He also pointed out the sub-divisional hospital in Dera Bassi, where staffing levels were deemed sufficient. However, the hospital faced issues related to space constraints. At the Zirakpur facility, Dr. Singh highlighted the availability of advanced medical services, including laparoscopic surgery, which is now being offered alongside other essential treatments.
Also Read: Over 2500 Punjab Govt doctors to resume strike on January 20 over unmet demands
The minister also addressed the ongoing issue of nursing staff shortages in the state’s hospitals. He explained that his visit was focused on assessing the situation firsthand by engaging with the public and offering important directives to the medical and paramedical staff to ensure the maintenance of high-quality patient care.
Powered by WPeMatico
Visakhapatnam: The Andhra Pradesh High Court has dismissed a writ petition challenging the newly adopted method for
conducting gap analysis of Common Bio-Medical Waste Treatment and
Disposal Facilities (CBWTFs) in the state. The Court held that such matters fall
under the expertise of specialised bodies, and judicial interference should be
limited.
Pointing out that such
cases require specialized knowledge or expertise, especially those made by
expert bodies or government agencies, the bench added, “This principle of
judicial restraint stems from the understanding that courts may lack the
specific technical or professional expertise necessary to adequately review
such decisions. Interference is usually only warranted when there’s clear
evidence of illegality, arbitrariness, or procedural impropriety.” The petition was filed by
a registered society comprising twelve members who operate CBWTFs across Andhra
Pradesh and work to raise awareness about proper biomedical waste management.
The petitioners argued
that the Andhra Pradesh Pollution Control Board (APPCB) was required to conduct
a gap analysis to assess the treatment capacity of existing CBWTFs over a
projected ten-year period. This would help determine if new facilities were needed
in any area, without affecting the coverage area of existing ones. However, the
petitioners claimed that APPCB was moving ahead to permit new CBWTFs without
completing a proper gap analysis, prompting several legal challenges in the
past.
In response, APPCB had
assigned the gap analysis task to the Andhra Pradesh Environment Management
Corporation Limited (APEMCL), which hired consultants to prepare reports. These
reports were later revised by a committee set up by APPCB. The Central Pollution
Control Board (CPCB) returned the reports with comments, noting deficiencies.
Although a revised study was carried out, the CPCB again found it
unsatisfactory and asked for a fresh report.
The petitioners also
contended that CPCB had adopted a new method for gap analysis, based on a study
from the South-East Asian region, without public consultation and contrary to
earlier approved guidelines. They referred to official letters from the Ministry
of Environment, Forest and Climate Change (MoEF&CC) and CPCB, which
required the gap analysis reports to be reviewed and approved by CPCB before
granting permission for new CBWTFs. Despite this, the petitioners claimed that
APPCB was still planning to approve new facilities, ignoring the deviations in
the updated gap analysis.
The society had submitted
representations to CPCB and APPCB highlighting these issues, but received no
satisfactory response, leading them to file the writ petition. The grievance of
the petitioner society is twofold. One is switching on to new methodology by
CPCB for conducting gap analysis study based on the study report of South-East
Asian region, instead of following old methodology, and the other is that APPCB
is not considering its representation dated 29.01.2025 submitted to consider
the gap analysis report without reference to any new methodology other than the
one that was adopted all over the country.
After considering the
submissions, a division bench of Chief Justice Dhiraj Singh
Thakur and Justice Ravi Cheemalapati observed, “It is needful to note that an inadequate number of treatment facilities and treatment facilities with
inadequate capacity to treat the waste generated may result in unscientific
disposal of bio-medical waste to the detriment of public health. The guidelines
and the methodology for conducting gap analysis must aim to ensure effective
treatment of bio medical waste for protection of environment and public health.”
“Therefore, the concerned Pollution Control
Boards must always strive to explore the new methods and modalities to narrow
down the gaps, if any, for ensuring compliance of the object of guidelines. Any
attempt to curtail them from switching on to new methodology based on studies
and adopting the methods followed by the nations across the globe would entail
derailment of the State’s Constitutional obligation for providing a pollution-free
environment and protection of natural environmental resources,” the court
further added.
Finally the court
dismissed the petition, pointing out that the Court did not find any patent
illegality, arbitrariness, or procedural impropriety in switching on the new
methodology by Central Pollution Control Board and advising the State Control
Boards to consider the recommendations made by them on gap analysis report
before concluding the requirement of new CBWTFs.
To view the order, click on the link below:
Powered by WPeMatico

Agartala: The Tripura government has initiated the recruitment process for 432 nursing staff, including 100 on a contract basis, to strengthen the healthcare services, announced Chief Minister Dr Manik Saha.
“About 153 nursing staff have been recruited in 2024. The state government has initiated the process to recruit 332 nursing staff to strengthen healthcare, and the necessary allocation has been received from the Finance Department to recruit 100 more personnel in the nursing profession on a contractual basis,” he said, news agency ANI reported.
CM Saha said this at an event organized on the occasion of International Nurses Day at Rabindra Shatabarshiki Bhavan in Agartala on Monday.
CM Saha, who holds the Health portfolio, also said that the International Nurses Day is celebrated all over the world on the birthday of the great woman Florence Nightingale.
Also Read:Odisha introduces new dress code for Nursing staff with distinct colours
He said, “We have to give importance to the health, safety, and well-being of those associated with nursing. And this time, this theme has been adopted to make the profession more attractive. Along with this, emphasis has also been placed on its economic importance. If quality care is enhanced through the nursing profession, a strong economy will also be possible.
Nursing is an important profession. When a patient goes to the hospital, nurses provide primary care before the doctors arrive. That is, they are the front runners. Nurses are the trust of the dying.”
Dr. Saha added that from checking blood pressure on time to administering medicines and giving injections, nurses take care of the patients.
“Usually, the number of patients is higher than that of nursing staff. In this case, it is being assessed how many nursing staff should be deployed compared to the number of patients. Those associated with nursing are working for the people.
You have the good fortune to serve people. Many children from Tripura are now going abroad by joining the nursing profession. We have to earn people’s recognition, respect, and admiration through relentless efforts and service.
And that is possible through good work. When patients return home after recovering, they always remember the doctors and nurses. Therefore, there is no room to underestimate this profession,” he said.
The Chief Minister further stated that in the financial year 2023-24, the Nursing Training Institute has been upgraded to Agartala Government Nursing College.
“Now all the facilities including BSc Nursing, BDS, MBBS courses have been made available in the state. Children from Tripura are now getting the opportunity to study various courses. The 40-seat ANM Nursing Institute in Udaipur has been upgraded to GNM,” said Dr. Saha.
Social Welfare and Social Education Minister Tinku Roy, Health Secretary Kiran Gitte, Health Director Dr. Tapan Majumder, Family Welfare and Disease Prevention Director Dr. Anjan Das, Medical Education Director Dr. H. P. Sharma, Tripura Nursing Council Registrar Rebecca Darlong, and other dignitaries were present as distinguished guests on the occasion.
Also Read:Registration to Delhi Nursing Council mandatory: Delhi AIIMS directs Nursing Staff
Powered by WPeMatico
