Is there a relation between stillbirth Risk in Grandchild and Grandmother’s Obesity?

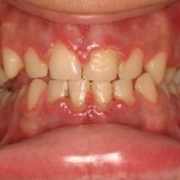
Women’s obesity and overweight problems have reached epidemic levels, making them the most significant preventable risk factors for stillbirth.
Eduardo Villamor, in a recent investigation published in the American Journal of Epidemiology, said grandmaternal overweight and obesity are associated with grand offspring stillbirth.
In this Swedish population-based study, researchers investigated the association between maternal grandmaternal early pregnancy BMI and grand offspring stillbirth risk. This three-generation cohort included nearly 176,908 grandmothers, 197,579 mothers, and more than 316,459 grand offspring born 1997-2016, using Swedish Medical Birth Register.
Key findings are:
- There were 998 stillbirths reported in grand offspring with risk, 3.2 per 1000 births.
- Compared to the grandmaternal normal BMI of 18.5-24.9 mg/kg2, stillbirth risk increased by 41 % (Relative risk 1.41).
- With increasing BMI ≥30, stillbirth risk increased by 62 % (relative risk 1.62).
- Maternal overweight and obesity in early pregnancy increased stillbirth risk in offspring by 32% and 77%, respectively.
- As indicated by causal mediation analysis, Maternal BMI mediated only 19% of this relation.
They said, In 101,368 pregnancies, we studied the relationship between maternal full sisters’ BMI and stillbirth risk to determine if shared familial factors explain the association. The stillbirth risk for full sisters with a BMI of 25.0-29.9 and ≥30 compared to 18.5-24.9 was 0.76 and 0.88, respectively. Our conclusion is that shared familial factors do not fully explain the association.
This study revealed an association between grandmaternal body mass index (BMI) and grand offspring stillbirth.
Observational design and selection bias due to missing data were the limitations of this study.
National Institutes of Health funded the study.
Reference:
Eduardo Villamor, Sven Cnattingius, Grandmaternal body mass index in early pregnancy and risk of grand offspring stillbirth: A nationwide, three-generation cohort study, American Journal of Epidemiology, 2023; kwad235, https://doi.org/10.1093/aje/kwad235
Powered by WPeMatico