New Delhi: For the first time, the Union Ministry of Health and Family Welfare has released an Expert Consensus Statement defining patients’ admission and discharge criteria to the Intensive Care Unit (ICU) and other related details.
These guidelines for Intensive Care Unit Admission and Discharge Criteria, compiled by a total number of 24 experts and released by the Directorate General of Health Services (DGHS) operative under the Union Health Ministry, addressed several issues including ICU Admission criteria and specifically it defined the critically ill patients who should not be admitted to the ICU.
Apart from this, these guidelines also clarified the ICU Discharge criteria, further defining the minimum patients monitoring required while awaiting an ICU bed, the minimum stabilisation required before transferring a patient to ICU, and the minimum monitoring required for transferring a critically ill patient (inter-facility transfer to hospital/ICU).
Referring to the Expert Consensus Statements, the guidelines mentioned, “The Expert Consensus statements have been made using the Delphi methodology to generate consensus. The Steering Group for Delphi process was SNM, RKM and PN who conducted the Delphi surveys using Google forms, prepared the Delphi statements and the reports. The Steering Group did not vote in Delphi surveys. The rest of the Experts voted anonymously over three rounds. Consensus was defined as achieved for an option when voted by 70% or more of the Experts. Stability was checked for all responses. The final statements were drafted from the MCQ responses that achieved consensus and stability.”
Expert Consensus Statement- Seven Things to Remember:
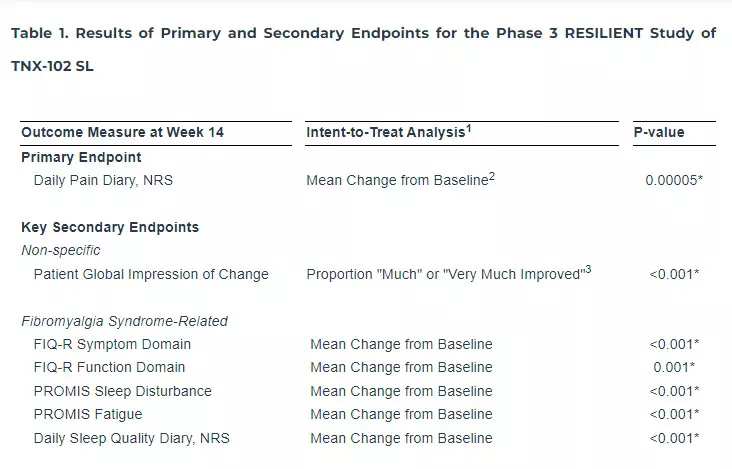
1. First of all, these guidelines clarified that the “Criteria for admitting a patient to ICU should be based on organ failure and need for organ support or in anticipation of deterioration in the medical condition.”
Other ICU-related details including admission, discharge, patient monitoring, and transfer of patients are as follows:
2. ICU Admission Criteria:
The guidelines lay down the following admission criteria to the Intensive Care Unit:
Altered level of consciousness of recent onset
Hemodynamic instability (e.g., clinical features of shock, arrythmias)
Need for respiratory support (e.g. escalating oxygen requirement, de–novo respiratory failure requiring non-invasive ventilation, invasive mechanical ventilation, etc.)
Patients with severe acute (or acute–on–chronic) illness requiring intensive monitoring and/or organ support
Any medical condition or disease with anticipation of deterioration
Patients who have experienced any major intraoperative complication (e.g. cardiovascular or respiratory instability)
Patients who have undergone major surgery, (e.g. thoracic, thoraco–abdominal, upper abdominal operations, trauma who require intensive monitoring or at a high risk of developing postoperative complications).
3. Patients who Should not be Admitted to the ICU:
Apart from clarifying the admission criteria for the ICUs, the new guidelines also clarified regarding the patients who cannot get admitted to the ICU. The details of patients who should not be admitted to ICU are as follows:
Patient’s or next–of–kin informed refusal to be admitted in ICU
Any disease with a treatment limitation plan
Anyone with a living will or advanced directive against ICU care
Terminally ill patients with a medical judgement of futility
Low priority criteria in case of pandemic or disaster situation where there is resourcelimitation (e.g. bed, workforce, equipment).
4. ICU Discharge Criteria:
Along with the admission criteria, the Union Government has also specified the criteria for getting a discharge from the ICU. The following patients or the following situations can fulfill the ICU Discharge Criteria:
Return of physiological aberrations to near normal or baseline status
Reasonable resolution and stability of the acute illness that necessitated ICU admission
Patient/family agrees for ICU discharge for a treatment-limiting decision or palliative care.
Based on lack of benefit from aggressive care (should be a medical decision, not obligating family agreement and as far as possible should not be based on economic constraints).
For infection control reasons with ensuring appropriate care of the given patient in a non ICU location
Rationing (i.e., prioritisation in the face of a resource crunch). In this event there should be an explicit and transparent written rationing policy that should be fair, consistent and reasonable.
5. The Minimum patient monitoring required while awaiting an ICU bed:
The Government has clearly mentioned in the guidelines regarding the minimum patient monitoring that is required while awaiting an ICU bed. These following factors should be monitored:
Blood pressure (continuous/intermittent)
Clinical monitoring (e.g., pulse rate, respiratory rate, breathing pattern, etc.)
Heart rate (continuous/intermittent)
Oxygen saturation – SpO2 (continuous/intermittent)
Capillary refill time
Urine Output (continuous/intermittent)
Neurological status e.g. Glasgow Coma Scale (GCS), Alert Verbal Pain Unresponsive (AVPU) scale etc.
Intermittent temperature monitoring
Blood sugar
6. The Minimum stabilisation required before transferring a patient to ICU:
As per the guidelines, the following aspects related to the stabilisation of a patient needs to be ensured before transferring a patient to the ICU:
Ensuring a secure airway (i.e., tracheal intubation if the patient has a GCS ≤8)
Ensuring adequate oxygenation and ventilation.
Stable haemodynamics, either with or without vasoactive drug infusion.
Ongoing correction of hyperglycemia/hypoglycemia and other life-threatening electrolyte/metabolic disturbances
Initiation of definitive therapy for life-threatening condition (e.g., external fixation of a fractured limb, administration of antiepileptics for recurrent seizures, antiarrhythmic drug infusion for unstable arrhythmias etc, intravenous antibiotics for sepsis)
7. The Minimum monitoring required for transferring a critically ill patient (inter-facility transfer to hospital/ICU):
The guidelines also specified the minimum monitoring that is required to transfer a critically ill patient. As per the new guidelines, the following conditions should be monitored:
Blood pressure (continuous/intermittent)
Clinical monitoring (pulse rate, respiratory rate, breathing pattern, etc.)
Continuous Heart rate
Continuous SpO2
Neurological status (AVPU, GCS, etc.)
The Government guidelines for admission of patients to ICU have been issued more than 7 years after the Supreme Court took cognisance of the issue. Medical Dialogues had reported back in 2016 that taking into account the stream of medical negligence cases being filed against the medical professionals and hospital, the Supreme Court bench had asked the Central Government, and the erstwhile Medical Council of India (MCI), which has now been replaced by the National Medical Commission (NMC), to answer whether any guidelines are prescribed for private hospitals on providing care to patients in the Intensive Care Unit (ICU) and Critical Care Unit (CCU).
Also Read: Give guidelines on admission to ICU,CCU: SC to MCI, Centre