Quick Review of Adult Vaccines with Dr Anantha Padmanabha

Powered by WPeMatico

Powered by WPeMatico

New Delhi: The drug major Eli Lilly has got a go-ahead from the Subject Expert Committee (SEC) functional under the Central Drug Standard Control Organisation (CDSCO) to import and market the antidiabetic drug Tirzepatide 2.5 mg/0.5 ml, 5mg/0.5ml, 10mg/0.5 ml, 12.5 mg/0.5 ml and 15mg/.5 ml solution for injection (single dose vial).
However, this approval is subject to the condition that the firm should conduct a Phase IV clinical trial and be required to submit the Phase IV clinical trial protocol to CDSCO within 03 months from the date of approval.
This came after the drug maker Eli Lilly presented the proposal for import and marketing permission of Tirzepatide 2.5 mg/0.5 ml, 5mg/0.5ml, 10mg/0.5 ml, 12.5 mg/0.5 ml and 15mg/.5 ml solution for injection (Single dose vial) along with their justification for bioequivalence (BE) and clinical trial (CT) waiver before the committee.
The firm informed that a similar formulation i.e Tirzepatide 2.5 mg/0.5 ml, 5mg/0.5ml, 10mg/0.5 ml, 12.5 mg/0.5 ml, and 15mg/.5 ml injection in the prefilled pen is already approved by the CDSCO for the same indication and now the firm has proposed for formulation in a single-dose vial presentation.
Tirzepatide is a dual GIP and GLP-1 receptor agonist used for the treatment of type II diabetes in adults as an adjunct to diet and exercise.
Tirzepatide is indicated as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus or for chronic weight management for adult patients that are obese or overweight with at least one weight-related comorbid condition such as hypertension, dyslipidemia, type 2 diabetes mellitus, obstructive sleep apnea, or cardiovascular disease).
Tirzepatide is a peptide molecule that acts as a dual agonist at GLP-1 and GIP receptors. It enhances the insulin response, suppresses glucagon secretion, promotes satiety, and improves insulin sensitivity.
At the recent SEC meeting for Endocrinology and Metabolism held on 13th and 14th February 2023, the expert panel reviewed the proposal for import and marketing permission of Tirzepatide along with their justification for BE and CT waiver.
After detailed deliberation, the committee recommended the import and marketing permission of Tirzepatide 2.5 mg/0.5 ml, 5mg/0.5ml, 10mg/0.5 ml, 12.5 mg/0.5 ml, and 15mg/.5 ml solution for injection (single dose vial) subject to the condition that the firm should conduct Phase-IV clinical trial.
Accordingly, the expert panel suggested that the firm should submit a Phase-IV clinical trial protocol to CDSCO within 03 months from the date of approval for further review by the committee.
Furthermore, the committee opined that the firm should fulfill the requirement of chemistry, manufacturing, and controls (CMC) data along with comparative data with that of the already approved pre-filled pen.
Also Read:Glenmark Gets CDSCO Panel Nod to Study ISB 2001 in Multiple Myeloma
Powered by WPeMatico
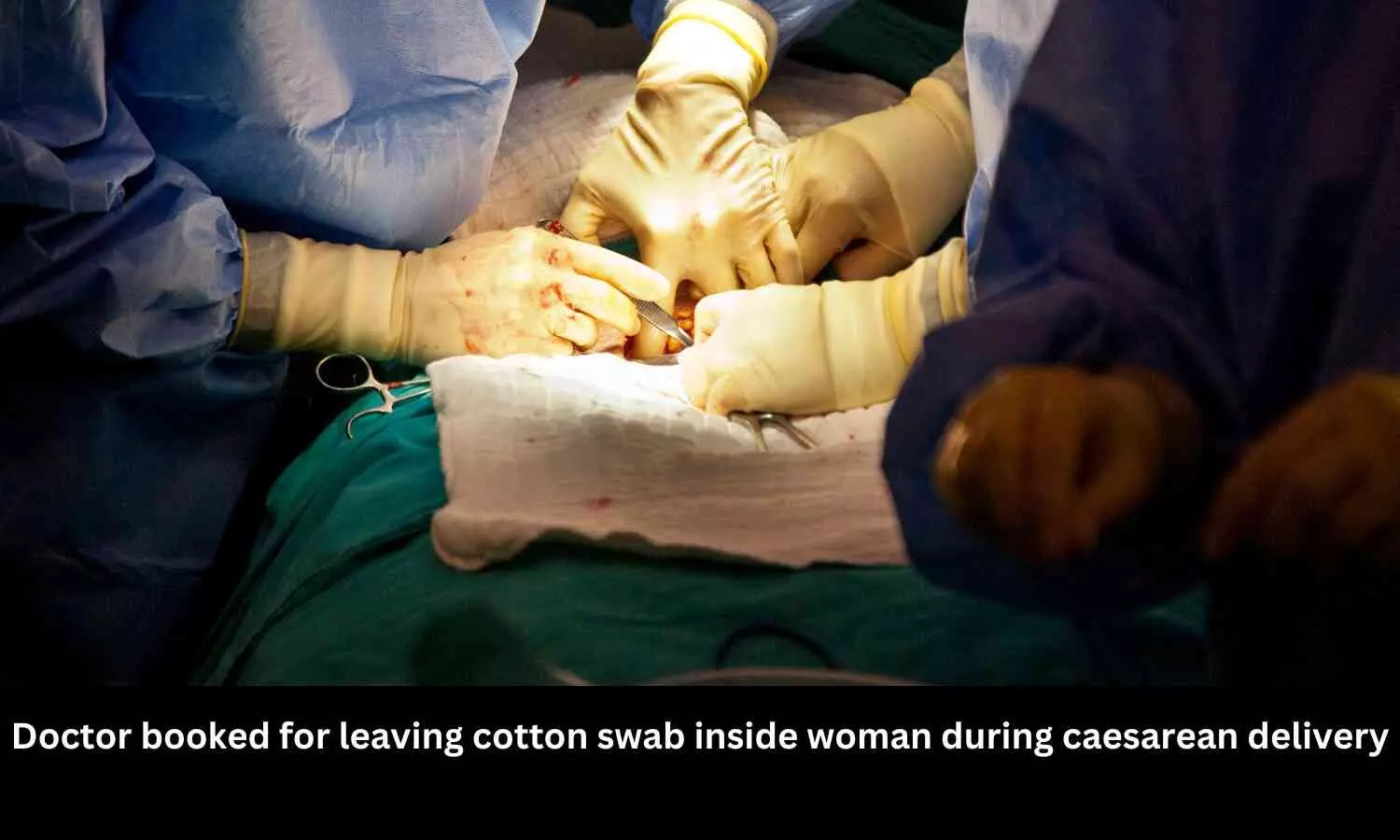
A doctor from a government hospital in Jambusar, Bharuch district, was booked for allegedly leaving a cotton swab inside a woman during a caesarean delivery last September. The woman, Amisha Solanki, underwent surgery at the sub-district hospital in Jambusar, operated by Dr. Charmi Ahir, where she gave birth to a boy. However, after discharge, she experienced stomach pain, leading to further medical consultations.
Powered by WPeMatico
Powered by WPeMatico
Powered by WPeMatico
Powered by WPeMatico
Powered by WPeMatico

Mumbai: Alkem Labs has announced that US Food and Drug Administration (US FDA) has issued an Establishment Inspection Report (EIR) for the Company’s API manufacturing facility located at Mandva.
The Inspection has been classified as Voluntary Action Indicated (VAI) and was closed accordingly.
Medical Dialogues team had earlier reported that Alkem Labs had received Form 483 with three observations from the USFDA at the end of the inspection conducted at the Company’s API manufacturing facility located at Mandva.
Read also: USFDA Issues 3 Observations For Alkem Labs Mandva Facility
Alkem Laboratories Ltd is an Indian pharmaceutical company engaged in the development, manufacture, and sale of pharmaceutical and nutraceutical products. The company produces branded generics, generic drugs, active pharmaceutical ingredients (APIs) and nutraceuticals in acute and chronic therapeutic areas, such as anti-infective, pain and analgesics, vitamins/minerals/nutrients, cardiac and Diabetology, Gynecology, Ophthalmology, neuro/central nervous system, dermatology, anti-diabetes, anti-osteoporosis, cardiovascular, and muscle relaxants, which are marketed in Indian and International markets. It operates through the pharmaceutical business segment.
Powered by WPeMatico
Powered by WPeMatico

Researchers at Indian Institute of Technology Madras(IIT Madras) and Translational Health Science and Technology Institute (THSTI), Faridabad, as part of ‘Interdisciplinary Group for Advanced Research on Birth Outcomes – DBT India Initiative’ (GARBH-Ini) program, have developed the first India-specific Artificial Intelligence (AI) model to determine the age of a foetus in a pregnant woman in the second and third trimesters precisely.
Powered by WPeMatico
