Treating Heart Failure with Anti-Obesity Medication: Dual Benefits for Clinical Outcomes and Climate Impact

New research has found that the use of GLP-1 receptor agonists*, a class of medications commonly prescribed for patients with obesity and diabetes, reduces the environmental footprint of healthcare and clinical outcomes when used to treat heart failure.
Treatment of heart failure patients with these therapies led to fewer hospitalizations and lower calorie intake, which together translated into decreased greenhouse gas emissions, less medical waste, and reduced water usage.
The study is one of the first to quantify the environmental co-benefits of pharmacologic treatment. The findings are being presented today at the 2025 European Society of Cardiology Congress in Madrid, Spain.
The healthcare sector is responsible for nearly 5% of greenhouse gas emissions globally (1), highlighting the need for interventions to reduce the environmental footprint of clinical care.
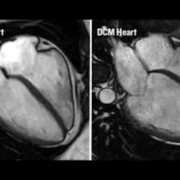
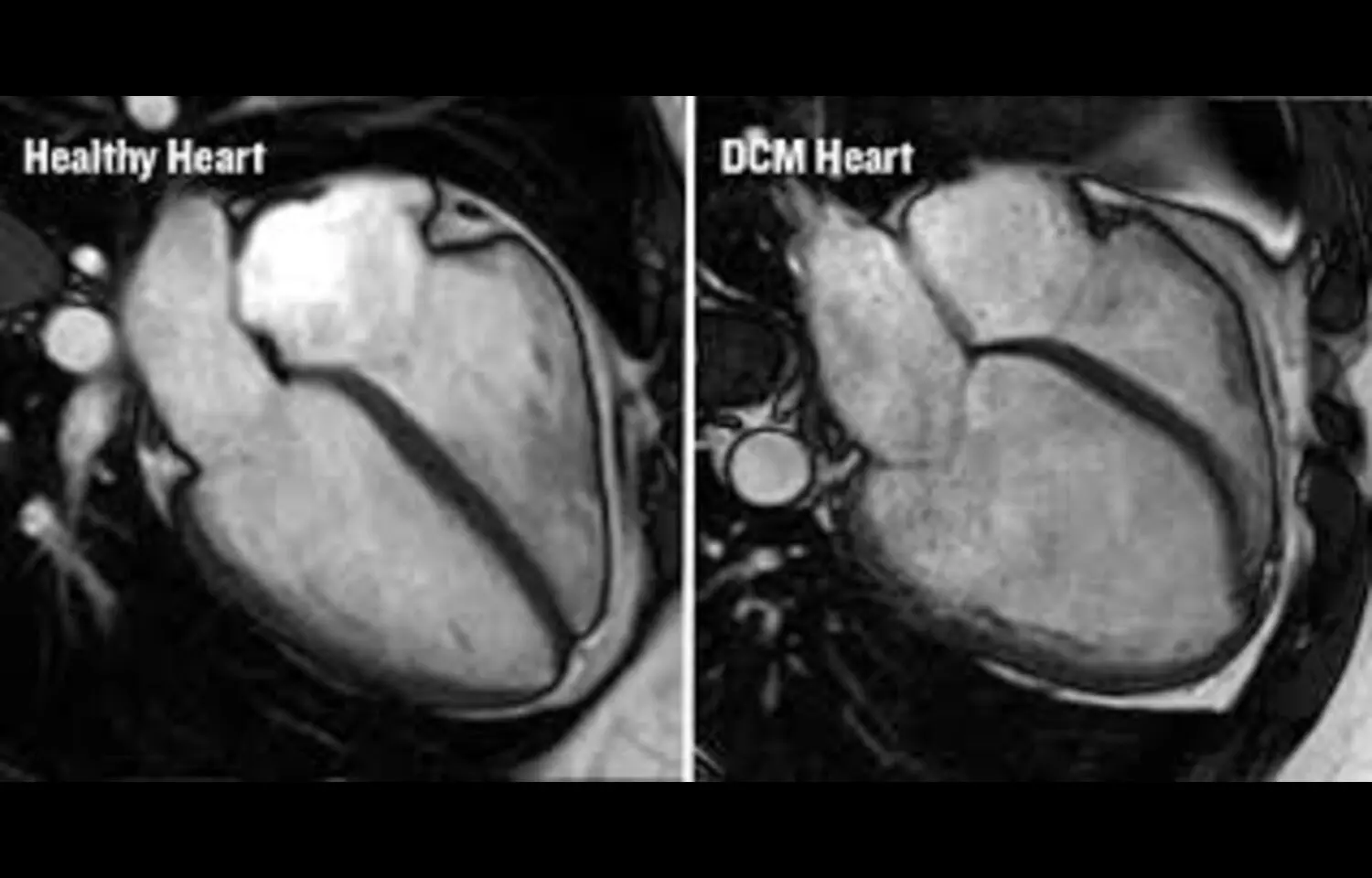
Patient level meta-analysis of four randomised controlled trials, SELECT, FLOW, STEP HFpEF, and STEPHFpEF DM, was conducted. All the trials involved patients with a type of heart failure, called heart failure with preserved ejection fraction, who had been treated with GLP-1 receptor agonists or a placebo. Estimates of mean inpatient days, ICU admissions, emergency department and ambulatory visits per event of heart failure, and related greenhouse gas emissions were obtained from previously published literature.
A total of 54 worsening heart failure events were reported among 1,914 patients receiving GLP1-receptor antagonist treatment, compared to 86 events among 1,829 patients receiving placebo.
Those receiving the treatment were estimated to have 9.45 kg CO₂-equivalent emissions per patient per year, compared to 9.70 kg CO₂-equivalent emissions per patient per year among placebo users. The differences were predominantly driven by emissions arising from inpatient stays and outpatient visits due to worsening heart failure events.
The study was led by Dr.Sarju Ganatra, Director of Sustainability and Vice Chair of Research at Lahey Hospital & Medical Center in the US and the President of Sustain Health Solutions, a non-profit organization that helps healthcare organizations integrate sustainability in care delivery. He explained, “The magnitude of the potential environmental emission savings found in our analysis was striking.”
“0.25 kg of CO2-equivalent per person saved annually from reduced hospitalizations might sound small. However, when this figure is scaled up to the millions of patients eligible for these therapies, it adds up to over 2 billion kilograms of CO2-equivalent saved. Similar-scale reductions were observed in waste generation and water use. This research highlights how even modest incremental individual gains can result in significant collective impact,” Dr Ganatra continued.
2 billion kg of CO2 is approximately equivalent to 20,000 full capacity Boeing 747 long-haul flights, or city-wide emissions from Brussels over 3 months. Around 30 million trees grown over 10 years would be needed to offset 2 billion kg of CO2.
The analysis also showed that those taking the GLP1-receptor antagonist treatment had approximately 695.33 kg CO2-equivalent lower emissions per patient per year due to a reduction in daily calorie consumption compared to placebo.
“By combining clinical trial data with environmental life cycle assessment metrics, we offer a new lens to evaluate the full impact of prescribing decisions. We also show that it is possible for medical treatments to deliver dual benefits-better health for patients and a healthier planet,” Dr Ganatra added. “We hope that in the future, policymakers will integrate sustainability metrics into health technology assessments, drug coverage decisions, and procurement frameworks.”
Each worsening heart failure event was assumed to involve one inpatient admission and a round trip to the hospital. Greenhouse gas emissions associated with the production and use of a GLP1 receptor agonist were obtained from a leading pharmaceutical drug manufacturer.
The study used modelling data from prior trials and established environmental life cycle assessment emissions data sets, rather than direct measurements. It could not take into account patient-level variability such as behavioural differences, and the research used mean values for hospital-related emissions.
“The next step for this research is to validate our modelling with real-world emissions data and clinical outcomes. In the future, we hope that environmental impact will be integrated into clinical trial designs, drug regulatory processes, and formulary decisions to ensure health systems align with planetary health goals,” Dr Ganatra concluded.
The findings from this study also support previous research that found that treatment of patients with GLP-1 receptor agonists reduced hospital visits for patients with heart failure with preserved ejection fraction.
Powered by WPeMatico