Health assessment tool gauges body’s biological age better than current methods
Powered by WPeMatico
Powered by WPeMatico
Powered by WPeMatico
Powered by WPeMatico
Powered by WPeMatico
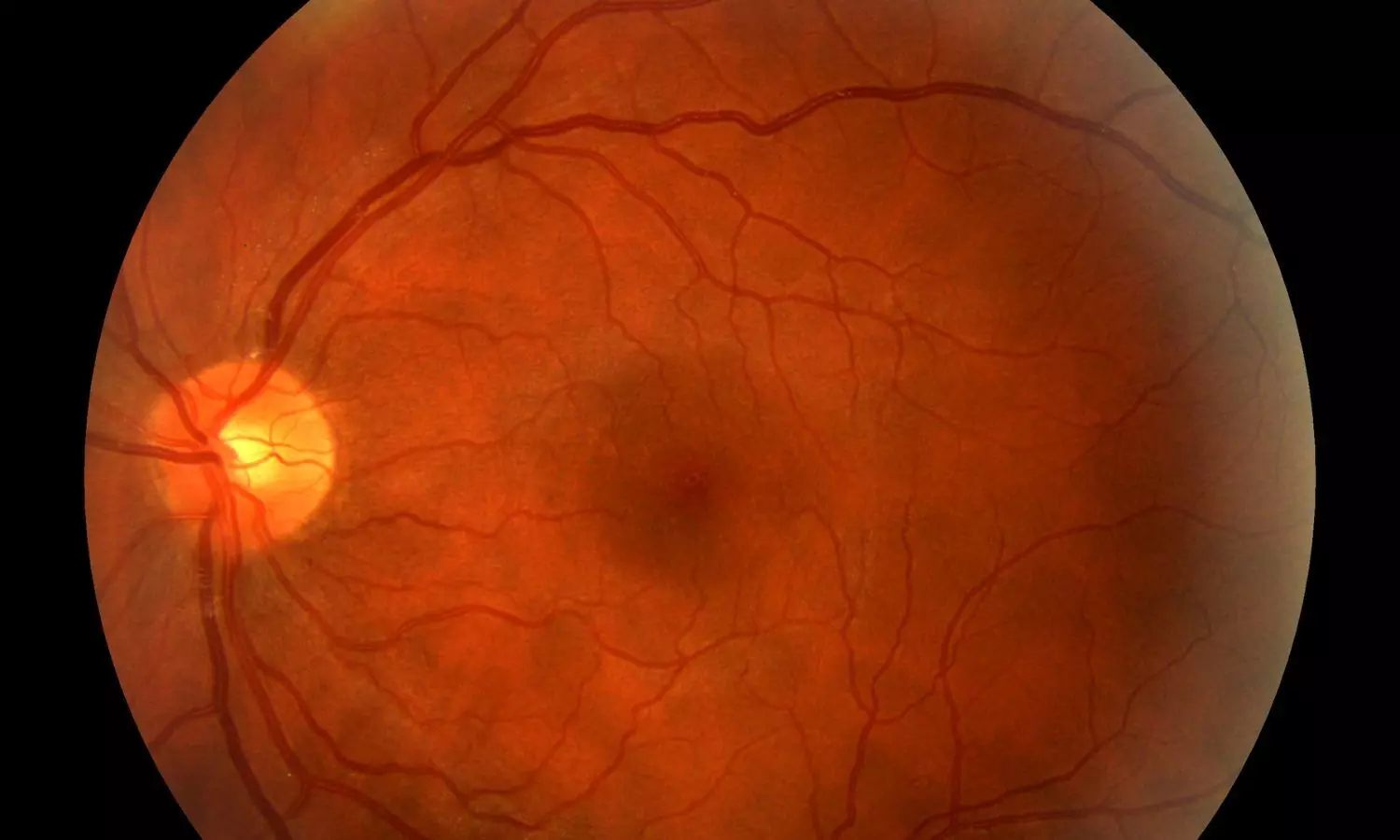
New Zealand: A new study published in Diabetes Technology & Therapeutics offers reassuring evidence that short-term use of automated insulin delivery (AID) systems is generally safe for diabetic retinopathy (DR) in people with type 1 diabetes, even amid rapid glycaemic improvements.
Diabetic retinopathy is a common and potentially serious complication of diabetes, and concerns have been raised that rapid improvements in blood glucose control, particularly in individuals with longstanding diabetes, may lead to early worsening of diabetic retinopathy (EWDR). Since automated insulin delivery systems are known to bring about swift and significant improvements in glycemic control, it becomes essential to evaluate their safety in this context.
To address this concern, Matilda M.E. Johansson, Department of Women’s and Children’s Health, University of Otago, Dunedin, New Zealand, and colleagues conducted a study to assess short-term outcomes of diabetic retinopathy in individuals aged 13 years and older with type 1 diabetes who had been using AID systems for a minimum of six months.
For this purpose, the researchers conducted a retrospective observational study across four centers, drawing participants from hospital databases in Dunedin and Christchurch, New Zealand, as well as from two research studies based in Auckland, New Zealand, and Perth, Australia. They gathered demographic and clinical information, along with diabetic retinopathy grading data before and after the initiation of AID, and performed statistical analyses to evaluate any changes in DR status over time.
The key findings of the study were as follows:
The study findings support the short-term retinal safety of automated insulin delivery (AID) systems in most individuals with type 1 diabetes, especially among younger users. The researchers reported that a majority of those who had recently begun using AID experienced either stable or improved diabetic retinopathy (DR) grades.
Highlighting a key insight, the researchers wrote, “Age at AID initiation under 18 years appears to offer a protective effect against the worsening of diabetic retinopathy.” Based on these observations, they concluded that early adoption of AID, before the onset of advanced age or multiple diabetes-related complications, may help preserve eye health and minimize the risk of DR progression in the short term.
Reference:
Johansson MME, March de Ribot F, Sime MJ, Boucsein A, Zhou Y, Jefferies CA, Paul RG, Wiltshire EJ, Abraham MB, Jones TW, de Bock MI, Wheeler BJ. Short-Term Diabetic Retinopathy Status in People with Type 1 Diabetes Commencing Automated Insulin Delivery. Diabetes Technol Ther. 2025 May;27(5):386-394. doi: 10.1089/dia.2024.0568. Epub 2025 Feb 10. PMID: 39925093.
Powered by WPeMatico
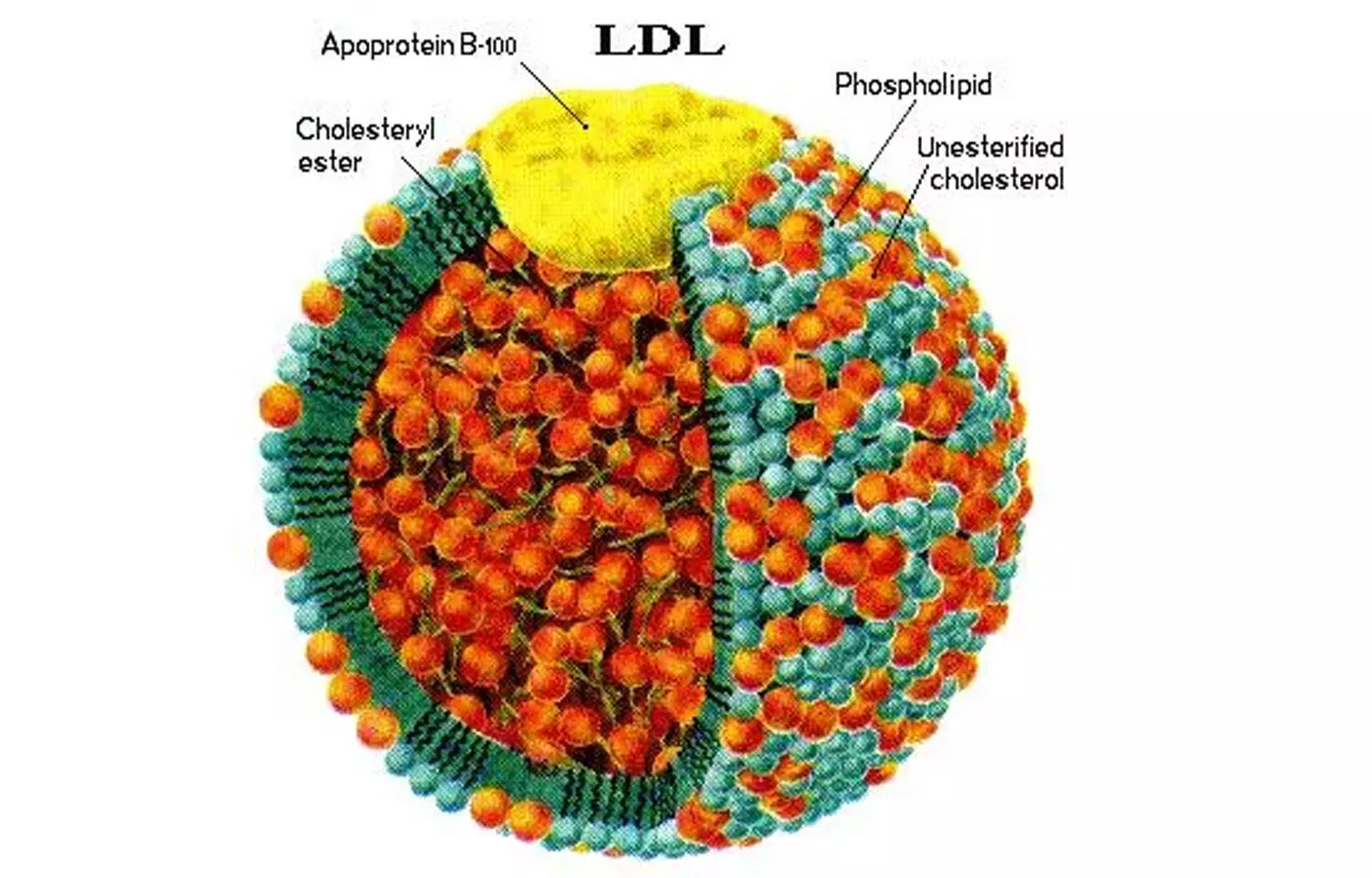
A recent study published in the European Heart Journal investigated lipoproteins containing apolipoprotein B, a protein essential to transporting “bad” cholesterol. The study concluded that the counts of apolipoprotein B particles and lipoprotein(a) are crucial indicators of lipid-related risk for coronary artery disease. This study was conducted by Jakub Morze and fellow researchers.
Apolipoprotein B (apoB) is found in all atherogenic lipoproteins, including very-low-density lipoprotein (VLDL) and low-density lipoprotein (LDL). Although apoB levels are already established as an important lipid marker, this research attempted to determine whether the particular type, size, or subclass of apoB-containing particles contributes anything to the predictive value for CAD over and above the total number. The contribution of lipoprotein(a), or Lp(a)—a genetically determined lipid marker—was also evaluated for its independent contribution to cardiovascular risk.
The study examined data for 207,368 UK Biobank participants who had no history of atherosclerotic cardiovascular disease, diabetes, or active lipid-lowering treatment at baseline. The researchers employed multivariable-adjusted Cox regression models to assess the association of various lipid measures with incident CAD. These were:
• Total apoB-P levels as quantified by nuclear magnetic resonance
• Individual lipoprotein class concentrations (LDL and VLDL)
• Size subclasses and mean particle diameter
• Lp(a) concentration as determined by immunoassay
The main outcome was the occurrence of coronary artery disease over the follow-up period.
Key Findings
• Each 1 standard deviation (SD) rise in total apoB-P was linked to a 33% higher risk of CAD (HR: 1.33; 95% CI: 1.30–1.36).
• While VLDL particles did have a greater per-particle CAD risk (HR per 100 nmol/L: 1.22; 95% CI: 1.11–1.34) than LDL particles (HR per 100 nmol/L: 1.07; 95% CI: 1.05–1.08), this was balanced by the observation that LDL contributed 91% of total apoB-P, whereas VLDL contributed only 9%.
• The risk per 1-SD increase was therefore 1.09 (95% CI: 1.05–1.14) for LDL and 1.24 (95% CI: 1.19–1.30) for VLDL.
• Particle size, diameter, or subclass did not demonstrate any significant relationship with CAD after controlling for apoB-P.
• Lipoprotein(a) concentration was still independently predictive of CAD after apoB-P adjustment, with a hazard ratio of 1.18 (95% CI: 1.16–1.20).
• Including Lp(a) in risk prediction models significantly enhanced prognostic accuracy, with the area under the curve (AUC) rising from 0.769 to 0.774 (P <.001).
This study concludes that total apoB-containing particle number (apoB-P) as the most accurate predictor of coronary artery disease risk, irrespective of particle size or type. Furthermore, lipoprotein(a) offers independent prognostic utility and must be factored into CAD risk evaluation. Combined, these markers provide an improved and more straightforward method for assessing atherosclerotic risk in clinical practice.
Reference:
Jakub Morze, Giorgio E M Melloni, Clemens Wittenbecher, Mika Ala-Korpela, Andrzej Rynkiewicz, Marta Guasch-Ferré, Christian T Ruff, Frank B Hu, Marc S Sabatine, Nicholas A Marston, ApoB-containing lipoproteins: count, type, size, and risk of coronary artery disease, European Heart Journal, 2025;, ehaf207,https://doi.org/10.1093/eurheartj/ehaf207
Powered by WPeMatico
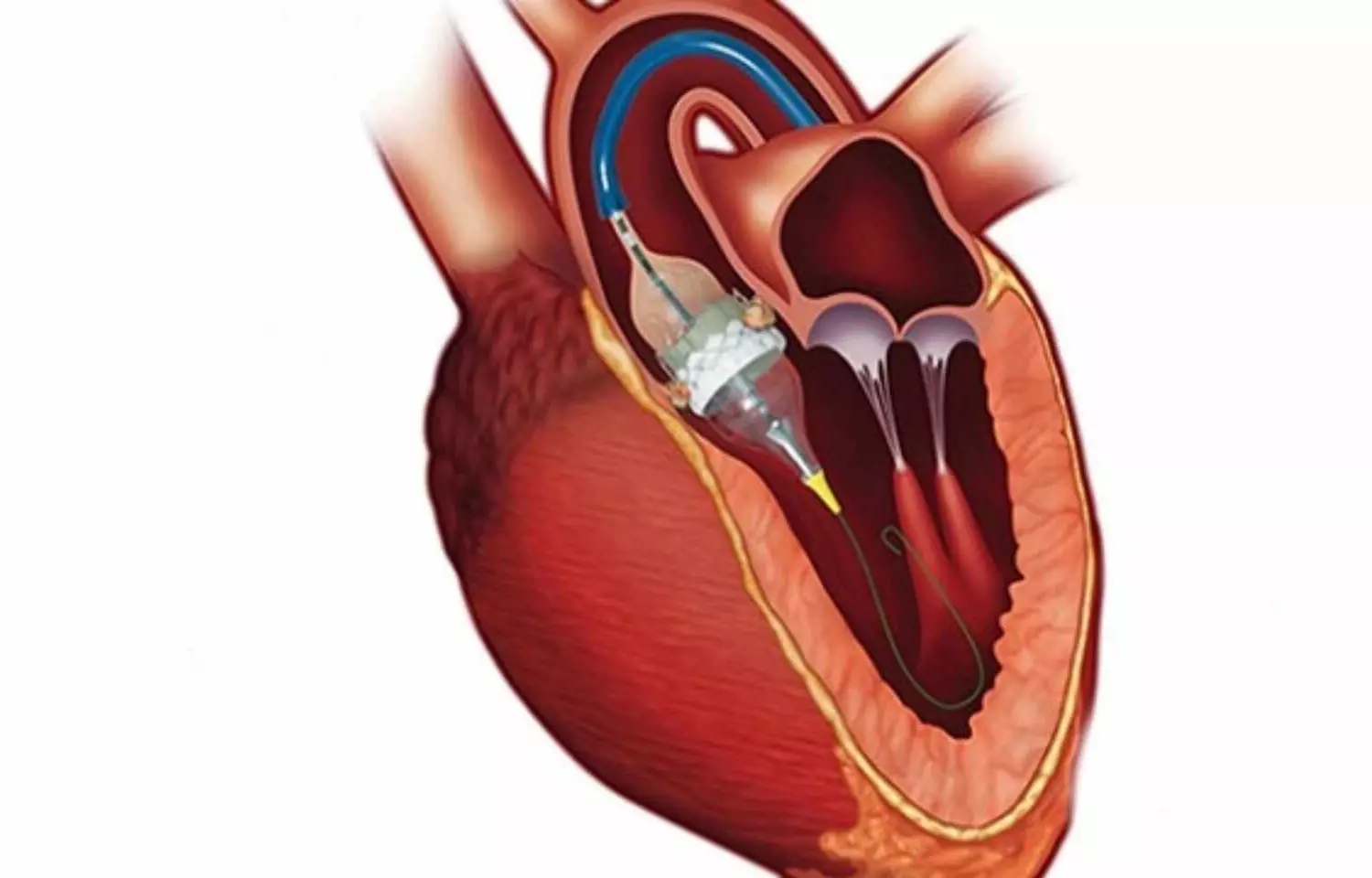
The US Food and Drugs Administration ( FDA) has approved the Sapien 3 transcatheter aortic valve replacement (TAVR) system for use in patients with severe aortic stenosis who are asymptomatic. This marks the first approval of a TAVR device for early intervention before symptoms appear, according to Edwards Lifesciences.
Approval of the SAPIEN 3 platform (SAPIEN 3, SAPIEN 3 Ultra and SAPIEN 3 Ultra RESILIA) is based on groundbreaking data from the EARLY TAVR trial, which demonstrated that asymptomatic severe AS patients randomized to Edwards TAVR experienced superior outcomes compared to guideline-recommended clinical surveillance (watchful waiting).
Without treatment, 1 in 10 patients experiencing symptoms of severe AS may die within five weeks. However, the symptoms of severe AS can be difficult to detect and may progress rapidly and unpredictably.
“There is an urgent need to change practice and TAVR guidelines for the treatment of aortic stenosis patients, which currently recommend ‘watchful waiting’ until symptoms develop,” said Philippe Genereux, M.D., director of the structural heart program at Gagnon Cardiovascular Institute, Morristown Medical Center, Morristown, New Jersey. “As we saw in the EARLY TAVR trial, patients originally designated as asymptomatic became symptomatic in sudden and unpredictable ways, underscoring the importance of early evaluation by a heart team to improve patient outcomes and benefit the healthcare system.”
The EARLY TAVR trial was the first randomized, controlled trial designed to evaluate TAVR compared to watchful waiting for patients with asymptomatic severe AS. With a median follow-up of 3.8 years, 26.8% of the 455 patients in the TAVR arm experienced death, stroke, or unplanned cardiovascular hospitalization, compared with 45.3% of the 446 patients in the clinical surveillance arm. The data were published last year in The New England Journal of Medicine (NEJM), marking the ninth NEJM publication on Edwards TAVR.
“This approval is a powerful opportunity to streamline patient care and improve the efficiency of the healthcare system,” said Larry Wood, Edwards’ corporate vice president and group president, Transcatheter Aortic Valve Replacement and Surgical. “We are proud to partner with leading physicians to advance our knowledge of this deadly disease with high quality science and optimize the treatment pathway for patients.”
Since its introduction more than two decades ago, SAPIEN has become the most studied valve platform, demonstrating unmatched clinical outcomes and solidifying its position as the leading choice for physicians and patients. More than 1 million patients have been treated with SAPIEN valves worldwide.
Powered by WPeMatico
Researchers at the University of Exeter have developed a tool to identify the most effective glucose-lowering drugs for individuals with type 2 diabetes. By predicting which medication will provide the greatest reduction in blood sugar levels, this user-friendly tool has the potential to improve health outcomes for millions.
Researchers at the University of Exeter, funded by the Medical Research Council, Wellcome and NIHR Exeter Biomedical Research Centre, and supported by Diabetes UK, have developed an innovative way of identifying the most effective glucose-lowering drugs for a person with type 2 diabetes. By predicting which drug will lead to the largest reduction in blood glucose levels, the easy-to-use tool could pave the way for better health for millions, at the push of a button.
Careful management of blood glucose levels is essential for reducing the risk of serious diabetes complications. However, keeping blood glucose levels in a safe range can be challenging, with only about a third of people with type 2 diabetes meeting targets2. With diabetes-related complications devastating lives and costing the UK healthcare system £6.2 billion every year3, there is an urgent need for new approaches to improve blood glucose management.
In England alone, more than three million people with type 2 diabetes use glucose-lowering drugs to manage their condition4. While metformin is the most common first treatment, five other major types of glucose-lowering drugs are available. However, their effectiveness varies widely from person to person and it has not been possible to determine the best glucose-lowering treatment for each patient – until now.
The new tool was created to tackle the challenge of which drug to choose after metformin. It was developed and tested using data from one million people with type 2 diabetes in the UK, linking GP and hospital records, with its accuracy verified with data from clinical trials.
The research revealed that only 18% of people with type 2 diabetes in the UK have been treated with the most effective glucose-lowering drug for them.
Modelling showed that starting people on the drug recommended by the new tool could lead to marked reductions in blood glucose levels (HbA1c) at one year, of around 5mmol/mol on average. Importantly, these improvements in blood glucose levels could approximately double the time until people need to start taking further diabetes medications. The tool’s use was also predicted to lower risks of developing serious long-term diabetes complications including heart attacks, strokes and kidney disease.
Using routinely collected clinical information, the tool offers a low-cost, practical, immediately usable solution that could transform the treatment of type 2 diabetes. For millions of people with type 2 diabetes, its use would ensure they receive the best treatment to help keep their blood sugars in target range and minimise their risk of developing life-limiting complications.
Dave Pomfrey, 67, from Hampshire, was diagnosed with type 2 diabetes 27 years ago. Dave was told he would need medication to manage his blood glucose levels and help reduce the risk of complications.
“The prospect of taking medication, potentially for life, along with the threat of complications was worrying. But having seen the impact of health complications faced by my relatives, who also had type 2 diabetes, I was willing to try anything.
“Over the years, I have experienced many changes to my medication to keep my blood glucose levels within range. Getting the most appropriate treatment as early as possible and reducing the back and forth of trying different medications could have such an impact on a person’s wellbeing.”
The tool’s performance in clinical practice is currently being assessed in 22,500 patients with type 2 diabetes across Scotland, which will inform its roll-out across the UK and globally, enabling a new era of personalised treatment for people with type 2 diabetes.
John Dennis, Associate Professor at the University of Exeter who led the study, said: “We have developed a completely new personalised approach for diabetes treatment, that could benefit everyone with type 2 diabetes in the UK and worldwide. For the first time, our model allows people living with type 2 diabetes to quickly identify the best treatment to manage their blood sugar levels, helping reduce their risk of diabetes complications. This offers a major advance on the current approach to choosing diabetes medications.”
Professor Andrew Hattersley from the University of Exeter, added: “Critically, our model can be implemented in clinical care immediately and at no additional cost. This is because it uses simple measures such as sex, weight and standard blood tests that are performed routinely. We hope that we can roll out the model quickly to make it available to help people with type 2 diabetes in the UK and across the world.”
Dr Elizabeth Robertson, Director of Research and Clinical at Diabetes UK, said: “This innovation using routine clinical data could help countless people with type 2 diabetes to get their blood sugars levels into a safe range, significantly reducing their risk of devastating diabetes complications and easing the burden of living with this relentless condition. If shown to be effective in practice and widely adopted by health services in the UK and globally, this tool could mark the most significant advance in type 2 diabetes care in more than a decade, improving health outcomes for millions.”
Dr Adam Babbs, Head of Translation at the Medical Research Council, which was a funder of the study, said: “This study is a trailblazing example of why we’re backing research into the next frontier in medicine: the development of precision medicine, which ensures that the right patient receives the right therapy at the right time. With large variation between individuals with type 2 diabetes in how they respond to treatments, and many different treatments available, precision medicine like this has huge potential to improve patient outcomes and provide efficiencies for the healthcare system.”
References: John M Dennis, PhDa j.dennis@exeter.ac.uk ∙ Katherine G Young, PhDa ∙ Pedro Cardoso, PhDa ∙ Laura M Güdemann, PhDa ∙ Andrew P McGovern, MDa ∙ Prof Andrew Farmer, DMb ∙ Prof Rury R Holman, FMedScic,d ∙ Prof Naveed Sattar, MDe ∙ Trevelyan J McKinley, PhDa ∙ Prof Ewan R Pearson, PhDf ∙ Prof Angus G Jones, PhDa ∙ Beverley M Shields, PhDa ∙ Prof Andrew T Hattersley, DMa on behalf of the MASTERMIND Consortium† DOI 10.1016/S0140-6736(24)02617-5
Powered by WPeMatico

Researchers have identified in a new study that an elevated hemoglobin glycation index (HGI) is independently linked with higher risks of 30-day and 365-day mortality in heart failure (HF) patients admitted to the ICU. This research, using data from the MIMIC-IV database (2008-2019), emphasizes the prognostic significance of HGI, highlighting its potential utility in the identification of high-risk patients. The results indicate that the inclusion of HGI in clinical practice may enhance risk stratification and patient outcomes. The study was conducted by Ziyu Guo and colleagues published in BMC Diabetology & Metabolic Syndrome.
HGI is the observed-predicted difference for hemoglobin A1c (HbA1c) values. HbA1c has gained popularity in the management of diabetes, but it has not yet been explained completely in the context of HF prognosis. The aim of this study is to define the association between HGI and mortality in HF patients who are admitted to the ICU.
This study was conducted with a retrospective analysis of data from the MIMIC-IV database. The patients included in the study were previously admitted to intensive care for HF. A linear regression model was formulated to estimate predicted HbA1c from fasting blood glucose (FBG). The HGI value was established by the observed minus the predicted HbA1c. The major endpoints were 30 and 365-day all-cause mortality after admission.
Kaplan–Meier survival curves were utilized to contrast mortality rates between various HGI groups. Cox regression models and restricted cubic spline (RCS) analysis were used to evaluate the association between HGI and mortality risk. Subgroup and sensitivity analyses were used to confirm the results.
Key Findings
• 2,846 HF patients (40.1% male) were included.
• 10.7% (305 patients) of them died within 30 days, and 33.5% (954 patients) of them died within 365 days.
• Kaplan–Meier analysis also showed that a high HGI was significantly correlated with higher risk of mortality (log-rank P < 0.001).
• Those who had high HGI were significantly at a higher risk of 30-day (adjusted hazard ratio [aHR]: 2.36, 95% CI: 1.74–3.20, P < 0.001) and 365-day (aHR: 1.40, 95% CI: 1.16–1.68, P < 0.001) mortality.
• Every one-point rise in HGI was associated with 1.42-fold increased risk of 30-day mortality (aHR: 1.42, 95% CI: 1.28–1.57, P < 0.001) and 1.19-fold increased risk of 365-day mortality (aHR: 1.19, 95% CI: 1.11–1.68, P < 0.001).
• RCS analysis revealed an L-shaped nonlinear relationship between HGI and mortality (P for nonlinearity < 0.001), with an inflection point at HGI = -1.295.
• A high HGI value (> 0.709) was identified as a threshold for increased risk of mortality.
The study authors concluded that in critically ill heart failure patients admitted to ICU, high hemoglobin glycation index was strongly correlated with short-term and long-term mortality risks. The results indicate that HGI has the potential to be a useful prognostic indicator in clinical practice, facilitating the detection of high-risk patients and the best treatment strategies to maximize survival rates.
Reference:
Powered by WPeMatico

Recent study investigated mother-to-child transmission (MTCT) rates of hepatitis B virus (HBV) in infants born to non-antiviral mothers with high viremia (HBV DNA > 2 × 10^5 IU/ml) under various delivery and feeding modes. Chronic HBV infection poses significant public health concerns, with MTCT being a primary transmission route. After the implementation of passive-active immunoprophylaxis, MTCT rates significantly decreased; however, additional factors like mode of delivery and choice of feeding remain contentious. The research involved secondary analysis from two multicenter prospective cohort studies, focusing solely on mothers with high viremia who did not receive antiviral therapy. A total of 462 infants, born to 460 mothers, were included. They were divided based on their delivery (vaginal delivery or elective cesarean section [ECS]) and feeding modes (breastfeeding or bottle-feeding). All infants received hepatitis B immunoglobulin and the hepatitis B vaccine shortly after birth.
Infection Rate Outcomes
Results indicated that among the 462 infants, the overall HBV infection rate was low at 2.4%. Infection rates were comparable across different delivery methods: 2.0% for vaginal deliveries and 2.8% for ECS. Likewise, no significant difference in infection rates was observed between breastfed (3.2%) and bottle-fed infants (1.1%). Upon analyzing various combinations of delivery and feeding modes, MTCT rates were also similar across these groups, suggesting that neither ECS nor bottle-feeding offered additional protection against HBV transmission.
Efficacy of Immunoprophylaxis
The findings emphasized the efficacy of timely immunoprophylaxis in reducing MTCT rates, aligning with previous studies that highlighted similar outcomes. Moreover, breastfeeding did not appear to elevate the risk of HBV transmission, supporting recommendations from major health organizations advocating for breastfeeding even in HBV-infected mothers.
Study Limitations
Despite the positive implications of the study, several limitations were noted. The non-randomized allocation of participants among groups and the absence of non-elective cesarean sections in the analysis limited the breadth of conclusions. Furthermore, the timing of immunoprophylaxis might influence external validity in settings where immediate vaccination is not guaranteed. Ultimately, the study concluded that vaginal delivery and breastfeeding are safe for infants born to high viremic HBV carrier mothers, reinforcing the need for maternal antiviral prophylaxis to effectively prevent MTCT of HBV.
Key Points
– The study assessed mother-to-child transmission (MTCT) rates of hepatitis B virus (HBV) in infants born to non-antiviral mothers with high viremia (HBV DNA > 2 × 10^5 IU/ml), highlighting the impact of delivery and feeding methods post-birth.
– A total of 462 infants born to 460 mothers were analyzed, with all infants receiving hepatitis B immunoglobulin and vaccination after birth. The MTCT rate was low at 2.4%, with similar rates observed across delivery methods (2.0% for vaginal and 2.8% for elective cesarean section) and feeding options (3.2% for breastfeeding and 1.1% for bottle-feeding).
– Detailed examination of various combinations of delivery and feeding modes revealed no significant differences in infection rates, indicating that elective cesarean sections and bottle-feeding did not provide additional protection against HBV transmission.
– The efficacy of timely immunoprophylaxis in reducing MTCT rates was demonstrated, supporting the continuation of breastfeeding in HBV-infected mothers as recommended by health organizations since it did not increase transmission risk.
– Study limitations included the non-randomized allocation of participants and the lack of analysis on non-elective cesarean sections, potentially restricting the applicability of the findings. Additionally, the timing of immunoprophylaxis may affect results in settings with delayed vaccination.
– The study concluded that vaginal delivery and breastfeeding are safe for infants born to mothers with high viremic HBV, while emphasizing the importance of maternal antiviral prophylaxis to effectively reduce HBV transmission to infants.
Reference –
Hongyu Huang et al. (2025). Elective Cesarean Section And Bottle-Feeding Do Not Reduce Infection Of Hepatitis B In Infants Of High Viremic Mothers: A Retrospective Study. *BMC Pregnancy And Childbirth*, 25. https://doi.org/10.1186/s12884-025-07606-z.
Powered by WPeMatico
