Low birthweight coupled with overweight in 20s linked with ‘massive risk’ of early type 2 diabetes in men
Powered by WPeMatico
Powered by WPeMatico
Powered by WPeMatico
Powered by WPeMatico

Bhopal: The Madhya Pradesh cabinet on Monday approved a proposal to facilitate the availability of government district hospitals to investors who want to establish medical colleges under the public-private partnership (PPP) model.
This will reduce the investment cost and the investor will only need to build the medical college and not a hospital along with it, state Urban Development Minister Kailash Vijayvargiya said.
A hospital is needed for a medical college and the state government has such facilities (hospitals) in every district, he said.
Also Read:Tripura open to setting up its 3rd medical college under PPP Mode
The decision was taken in the cabinet meeting chaired by Chief Minister Mohan Yadav, he said.
For the establishment of medical colleges under the PPP model, the government will provide land at the collector guideline rates to investors, he said.
It is considered that establishing a hospital and the medical college together cost around Rs 500 crore to the investors, the minister said.
“The government will give the district hospital to the investor in a bid to reduce the investment cost. So, the investor doesn’t need to construct a hospital and will only need to build the medical college,” he said.
The condition laid in this model is that the investor will pay salaries of the district hospital staffers, Vijayvargiya said.
When the medical college and hospital start running together, then 75 per cent of the beds will need to be provided only to the poor and the investor can use the remaining 25 per cent commercially, he said.
“This is a very important decision and it will strengthen the medical infrastructure and improve facilities. We discussed this issue for about an hour,” the minister said, adding the government wants medical colleges in every district.
This scheme will be implemented in the districts where medical colleges are not there at present, he said.
The cabinet also took several other decisions, including implementation of the Centre’s Smart City 2.0 scheme.
Under the scheme, two-three cities of the state will be selected and an amount of about Rs 135 crore will be allocated to these cities for the improvement of environment, the minister said.
Powered by WPeMatico

New Delhi: Dr Mansukh Mandaviya, Union Minister of Health and Family Welfare yesterday launched the AYUSH, ICMR Advanced Centre for Integrated Health Research in AIIMS, here.
He also announced other mega joint initiatives between the Ministry of Health and Family Welfare and the Ministry of AYUSH which included Multicentre clinical trial on Anaemia and the launch of Indian Public Health Standards (IPHS) for AYUSH healthcare facilities.
The Union Health Minister also inaugurated the 27th convocation of Rashtriya Ayurveda Vidyapeeth and the 29th National Seminar on ‘Ayurvedo Amritanam’ on the occasion.
Expressing his elation at the launch of these collaborative initiatives, Dr Mandaviya said that, “Collaborative Research in AYUSH is extremely important as it bridges the gap between traditional knowledge and modern scientific research, promoting a synergistic approach to health care”. “Ayurveda is a part of our culture, heritage and tradition. It is still being followed in our everyday practice.
Also Read:ICMR Invites applications for a 4-week training course on Human Disease Models, details
This strategic collaboration aims to advance Integrative Health Research, integrate traditional AYUSH practices with modern medical science and take India to the forefront of holistic healthcare innovations”, he said.
Highlighting that the government is following an integrative approach so as to take the best practices from both the disciplines, Ayurveda and Allopathy, the Union Health Minister said that “The Union Government is working towards providing quality-oriented healthcare towards the needs of the people of the country. In this direction, Indian Public Health Standards (IPHS) were published by the Union Health Ministry as a set of uniform standards to improve the quality of health care delivery.
By adopting these reforms, it is expected that the States/UTs will be able to develop AYUSH health care services with set standards and quality infrastructure, thereby enabling the public to avail the benefits of AYUSH medical services for all healthcare.
The Union Health Minister congratulated the Ministry of AYUSH for its remarkable journey in the last decade which has resulted in significant initiatives and achievements. He also urged the students to inspiration from our ancient scriptures and follow their practices with pride.
Integrative Medicine (IM) is an approach to medical care that recognizes the benefit of combining conventional/modern medicine therapies with complementary and alternative medicine (CAM) therapies that have been shown to be safe and effective to promote holistic health considering individual’s requirements. IM also addresses the barriers to healing and provide the patient with the knowledge, skills and support to take better care of their physical, emotional, psychological and spiritual health. Rather than limiting treatments to a specific specialty, IM uses the safest and most effective combination of approaches and treatments from the world of conventional and complementary/alternative medicine.
There is a need for an integrated healthcare regime that can guide health policies and programs in the future. India has an advantage in this global resurgence of interest as it has a rich heritage of indigenous medical knowledge coupled with a robust infrastructure and skilled workforce in contemporary medicine. Recently, a Memorandum of Agreement (MoA) between M/o AYUSH and Indian Council of Medical Research (ICMR) was signed at an inter-ministerial level.
This is to promote high-impact research on Integrative Health to generate evidence in priority areas of national importance in healthcare utilizing modern scientific methods. This is a joint effort to establish AYUSH-ICMR Centers for Integrative Health Research (AI-ACIHR) at AIIMS in phases. Joint efforts will be in place to conduct high-quality clinical trials on identified areas/disease conditions of national importance with promising integrative therapies to generate evidence for wider acceptance.
AYUSH-ICMR Advanced Centre for Integrative Health Research are being set up to develop integrative health research through integrating AYUSH system with conventional bio-medicine, and modem technology to bring integrative health care to the people for improved patient outcome through innovations related to diagnostics, preventive, health promotive as well as treatment methods.
1. Advanced Centre for Integrative Health Research in Gastro-intestinal Disorders
2. Advanced Centre for Integrative Health Research in Women and Child Health
AIIMS Jodhpur: Advanced Centre for Integrative Health Research in Geriatric Health
AIIMS Nagpur: Advanced Centre for Integrative Health Research in Cancer Care
AIIMS Rishikesh: Advanced Centre for Integrative Health Research in Geriatric Health
CCRAS under Ministry of AYUSH and ICMR has undertaken a research study on Anaemia entitled “Efficacy and safety of Punarnavadi Mandura alone and in combination with Drakshavaleha compared to iron folic acid in the treatment of moderate iron deficiency anaemia among non-pregnant women of reproductive age group”: a community-based three arm multicenter randomized controlled trial. The study will be carried out at 8 different sites namely MGIMS Wardha, AIIMS Jodhpur, NITM Bengaluru, RIMS Ranchi, KEM Hospital Research Centre, AIIMS New Delhi, AIIMS Bhopal, and AIIMS Bibinagar.
The Indian Public Health Standards for AYUSH healthcare facilities aims to lay down uniform standards & quality infrastructure, human resource, medicines etc. By adopting these standards, States and UTs will able to extend quality AYUSH health care services to the deserving population. The fundamental aim is to augment preventive, promotive, curative, palliative, and rehabilitative services within the public health domain, emphasizing uncompromising quality. A pivotal principle within the National Health Policy (NHP) 2017 is the incorporation of Pluralism.
Vaidya Rajesh Kotecha, Secretary, Ministry of AYUSH; Smt. Anu Nagar, Joint Secretary, Health Ministry; Dr M Srinivas, Director, AIIMS New Delhi; Dr Goverdhan Dutt Puri, Executive Director, AIIMS Jodhpur; Prof. Rabinarayan Acharya, DG, Central Council for Research in Ayurvedic Sciences (CCRAS), Ministry of AYUSH; Vaidya Devendra Triguna, Ex-President, Governing Body, Rashtriya Ayurveda Vidyapeeth, New Delhi and senior officials of the Union Health Ministry and Union AYUSH Ministry were present on the occasion.
Powered by WPeMatico

Opening doors to convenience for patients suffering from
endometriosis, Mankind Pharma, a leading Indian pharmaceutical company, has
announced the launch of Dydroboon 30mg Sustained Release tablets in
India.
Dydroboon 30mg is approved by DCGI for the treatment of
endometriosis. It contains Dydrogesterone 30 mg in the sustained-release tablet
dosage form.
Women suffering from
endometriosis have to consume 2-3 pills of Dydrogesterone daily, increasing
their pill burden. Dydroboon 30mg sustained release tablets ensure drug release
for a longer time, mitigating this practical burden of treatment inconvenience.
It increases the chances of treatment adherence and helps the patient with the
convenience of once daily dose of the drug.
Dydrogesterone is a synthetic progestogen that works
similarly to progesterone. It addresses menstrual disorders arising from
hormonal imbalances in women. Dydroboon Tablet effectively treats various
conditions caused by progesterone deficiencies, such as female infertility,
pain during menstruation, premenstrual syndrome (PMS), endometriosis, abnormal
uterine bleeding, and miscarriage.
Commenting on the launch Mr. Rajeev Juneja, Vice-Chairman
& Managing Director, Mankind pharma said “Our commitment to patient
well-being drives our innovations. The launch of Dydroboon 30mg Sustained
Release Tablets reinforces our mission to enhance the lives of millions of women
battling endometriosis. As the 1st Indian company and 2nd in the world to
launch Dydrogesterone for Indian patients, we continuously focus on providing
women with treatment solutions that not only provide clinical efficacy but also
offer convenience, freeing patients from the daily challenge of managing
multiple pills.”
Powered by WPeMatico

Viking Therapeutics has unveiled compelling Phase 2 data for their dual GLP-1/GIP receptor agonist, VK2735 for chronic weight management. The VENTURE trial demonstrated promising results and thereby ignites hopes for a new therapeutic option for patients struggling with obesity.
This randomized, double-blind, placebo-controlled study assessed VK2735 across various dosages relative to placebo therapy. Over 13 weeks, 176 patients with obesity or overweight, along with weight-related comorbidities participated in this study. The percent change in body weight from baseline to week 13 was the primary endpoint which was met across all dosage groups.
Also, the patients receiving VK2735 15 mg achieved a remarkable mean placebo-adjusted body weight reduction of 13.1% by week 13, with an impressive 88% of individuals experiencing a placebo-adjusted weight reduction of 10% or more. These results underscore the potential of VK2735 as a game-changer in combating obesity.
The CEO of Viking Therapeutics, expressed enthusiasm over the outcomes of the trial highlighting the promising efficacy and tolerability profile of VK2735. Despite a 20% discontinuation rate among VK2735 recipients, safety analysis revealed mostly mild to moderate drug-related adverse events, with nausea being the most prevalent.
Viking Therapeutics outlined plans for further development of VK2735, including discussions with the FDA and the release of Phase 1 data for an oral formulation of the drug in the upcoming weeks. The CEO affirmed the commitment of the company to advance this vital therapy by signaling optimism for its progression into later clinical stages.
The positive results from the VENTURE trial mark a significant step in the pursuit of effective treatments for obesity and related comorbidities. VK2735 demonstrates promising efficacy and manageable safety profile to address the unmet needs in chronic weight management for patients seeking alternative therapeutic options.
Reference:
Viking therapeutics announces positive top-line results from phase 2 VENTURE trial of dual GLP-1/GIP receptor agonist VK2735 in patients with obesity. (n.d.). Viking Therapeutics InvestorRoom. Retrieved March 1, 2024, from https://ir.vikingtherapeutics.com/2024-02-27-Viking-Therapeutics-Announces-Positive-Top-Line-Results-from-Phase-2-VENTURE-Trial-of-Dual-GLP-1-GIP-Receptor-Agonist-VK2735-in-Patients-with-Obesity
Powered by WPeMatico
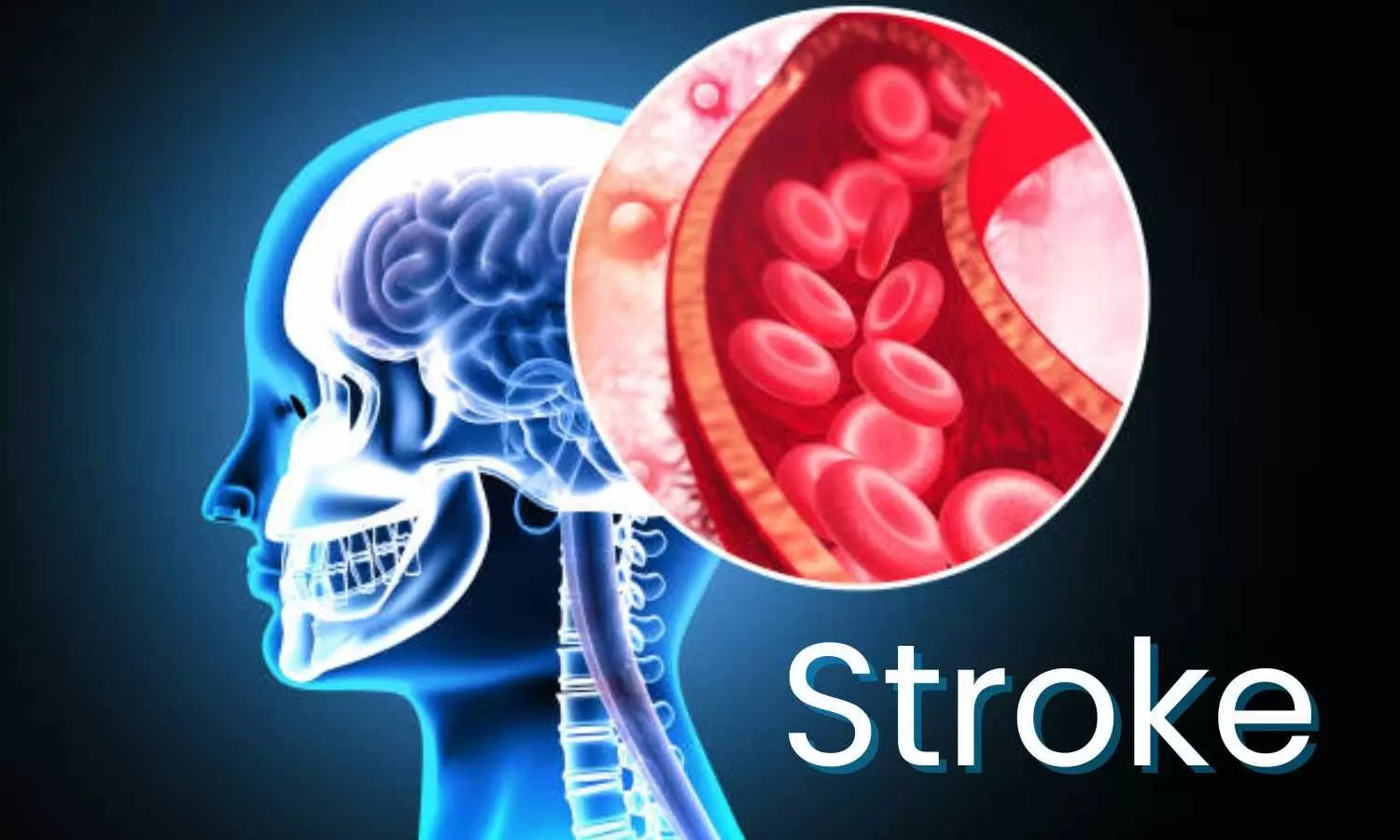
In a groundbreaking study conducted across more than 200 stroke centers in China, researchers have uncovered a direct and immediate association between hourly high ambient temperatures and the onset of Acute Ischemic Stroke (AIS). While previous studies have explored the broader connection between daily high temperatures and ischemic stroke outcomes, this research uniquely focuses on the hourly nuances of temperature exposure and its impact on AIS. The study’s outcomes suggest a significant association between hourly heat exposure and an increased risk of AIS onset.
The study results were published in the journal JAMA Network Open.
The study, structured as a time-stratified case-crossover, aimed to evaluate the association between hourly high ambient temperatures and the onset of AIS. Using a nationwide registry that compiled data from over 82,000 adult AIS patients hospitalized during warm seasons between 2019 and 2021, the research provided a granular examination of temperature exposure in the 24 hours leading up to the stroke onset.
Key Findings:
The study’s findings hold profound implications for public health strategies, particularly in the context of global warming. Understanding the immediate hourly dynamics of heat exposure and its correlation with AIS onset provides a valuable framework for targeted interventions. The research underscores the importance of adapting public health initiatives to address the specific challenges posed by rising temperatures, ensuring a proactive approach to mitigating the increased risk of AIS associated with ambient heat. As further studies delve into this critical area, these findings pave the way for informed strategies to protect individuals from the cerebrovascular risks linked to elevated temperatures.
Further reading: Zhu X, Chen R, Yuan J, et al. Hourly Heat Exposure and Acute Ischemic Stroke. JAMA Netw Open. 2024;7(2):e240627. doi:10.1001/jamanetworkopen.2024.0627
Powered by WPeMatico
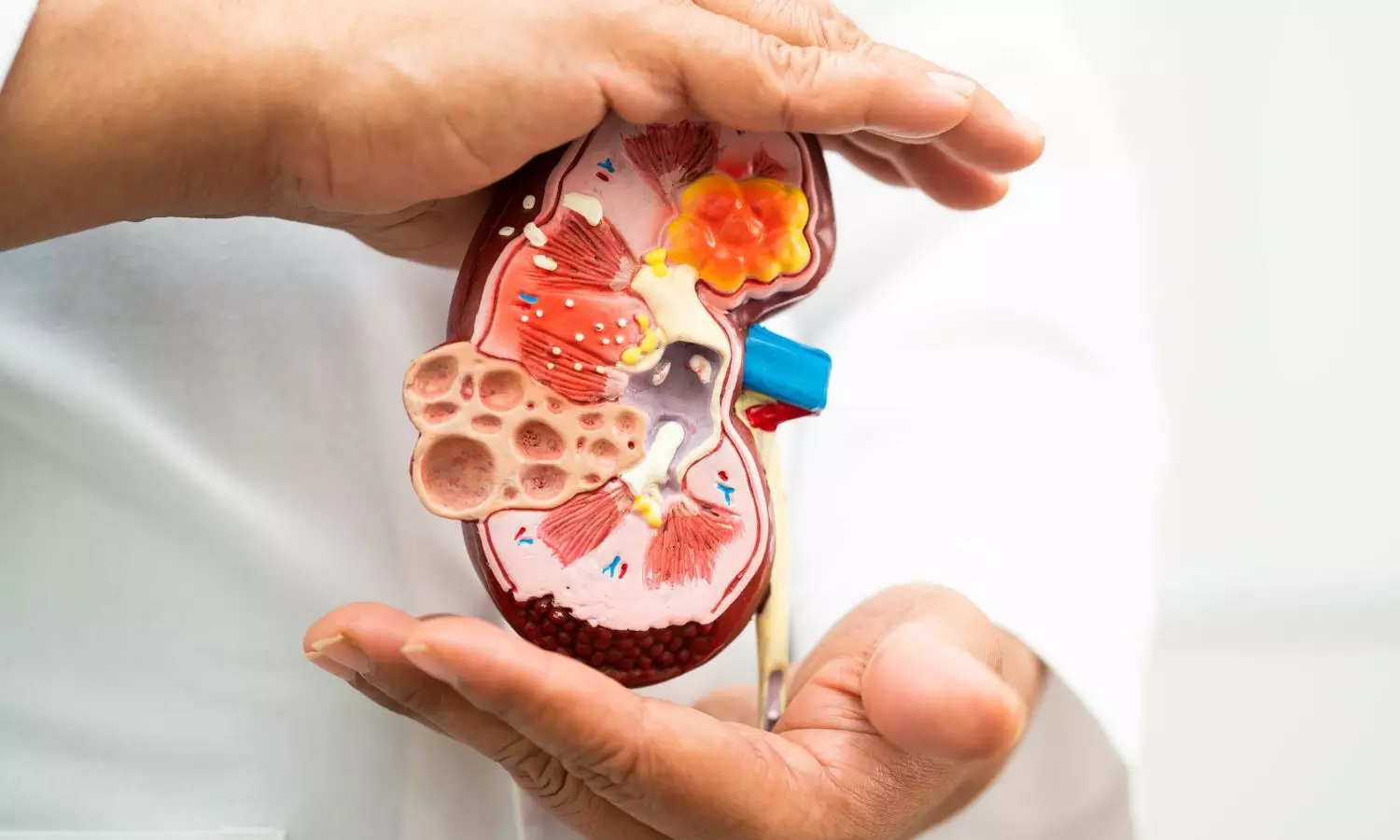
Chronic kidney disease (CKD) is a major health concern worldwide, affecting over 800 million people. As CKD is irreversible, lifestyle changes are crucial to prevent it. Dietary modifications play a vital role in managing and preventing CKD. Beverage intake, an essential component of dietary intake, can impact fluid balance, nutrient intake, and metabolic pathways. However, the relationship between beverage consumption and CKD risk remains unclear.
Powered by WPeMatico
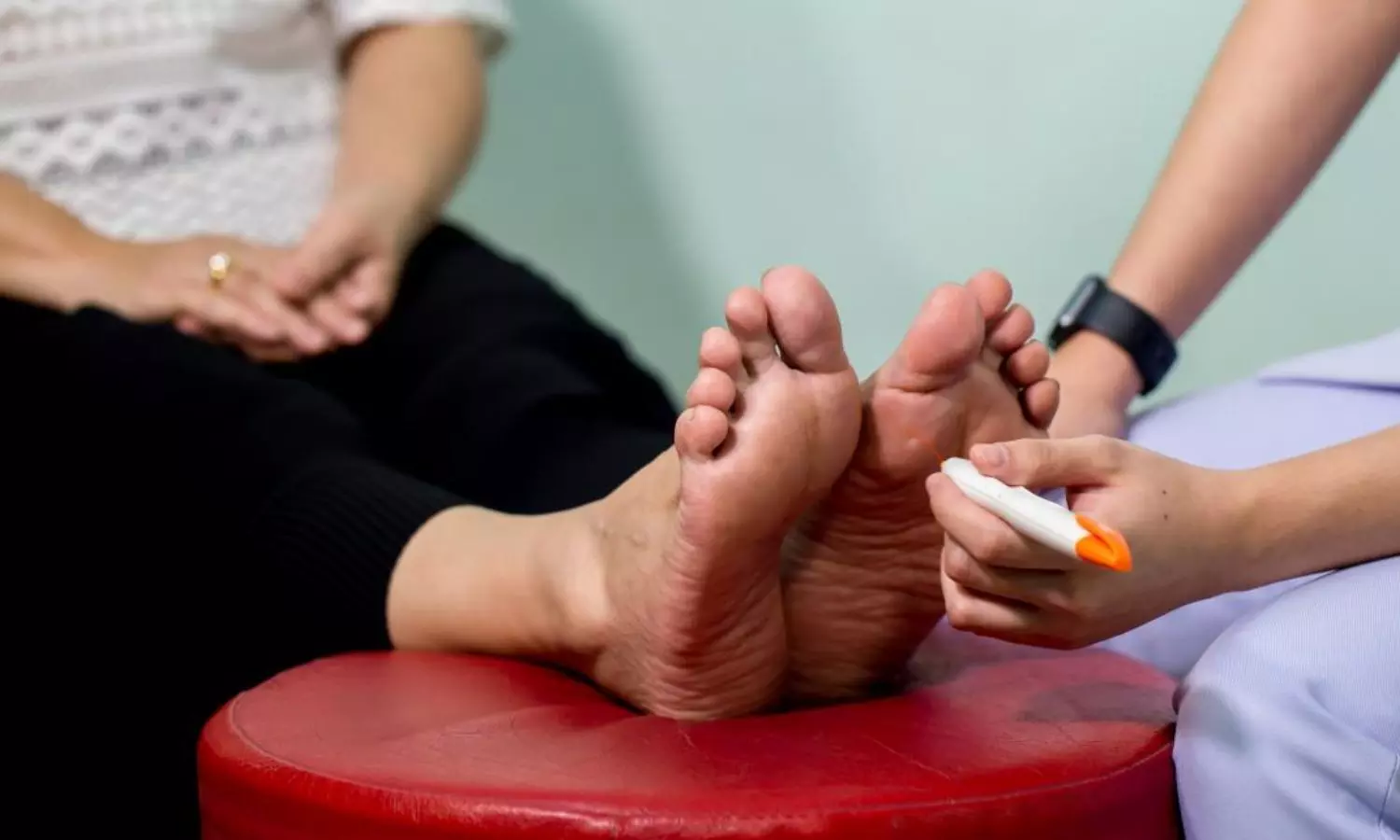
In a groundbreaking investigation, researchers have delved into the profound interconnection between Vitamin D deficiency (VDD) and diabetic peripheral neuropathy (DPN) among elderly individuals grappling with type 2 diabetes mellitus (T2DM). This study, involving 257 elderly T2DM patients, presents compelling insights into the intricate relationship between Vitamin D levels and the development of peripheral nerve complications. It underscores the independent association between Vitamin D deficiency and an elevated risk of diabetic peripheral neuropathy in elderly individuals with type 2 diabetes mellitus.
The study results were published in the journal Diabetes Research and Clinical Practice.
The research meticulously employed propensity score matching to ensure a balanced representation across age, sex, and diabetes duration among the participants. Vitamin D deficiency, marked by serum 25-hydroxyvitamin D [25(OH)D] levels below 20 ng/ml, emerged as a focal point for scrutiny. Assessments included electromyogram evaluations for large nerve fiber lesions and skin conductance measurements for small nerve fiber lesions.
Findings:
These findings underscore the independent association between Vitamin D deficiency and an escalated risk of diabetic peripheral neuropathy in the elderly T2DM population. The study suggests that Vitamin D deficiency may play a pivotal role in fostering the development of diabetic peripheral neuropathy, particularly by influencing large nerve fibers. As researchers unravel the intricate dance between Vitamin D and nerve health, these findings hold promise for targeted interventions and further research. Understanding the significance of maintaining optimal Vitamin D levels may prove instrumental in the comprehensive care and management of diabetic complications, offering a ray of hope for improved health outcomes in the aging population grappling with type 2 diabetes mellitus.
Further reading: Vitamin D deficiency increases the risk of diabetic peripheral neuropathy in elderly type 2 diabetes mellitus patients by predominantly increasing large-fiber lesions. DOI: https://doi.org/10.1016/j.diabres.2024.111585
Powered by WPeMatico
