U-shaped Kirschner wire internal fixation safe and effective treatment for Skier’s thumb: Study

U-shaped Kirschner wire internal fixation safe and effective treatment for Skier’s Thumb suggests a new study published in the BMC Surgery.
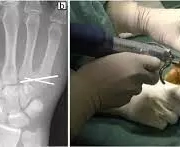
Skier’s thumb is a type of injury to the ulnar collateral ligament of the metacarpophalangeal joint of the thumb, which can result in bone fragmentation and joint instability. The objective of this study was to compare the traditional Kirschner wire fixation method with the U-shaped Kirschner wire method for treating small bone fragments with displacement, rotation, or instability in skier’s fractures. A retrospective study was conducted on 30 patients with skier’s thumb who were treated at Tianjin Hospital from January 2019 to December 2021. Patients were divided into two groups: Group A received traditional Kirschner wire fixation, while Group B received U-shaped Kirschner wire fixation. Functional assessments and complications during the perioperative period were evaluated. Results: Both surgical methods significantly reduced postoperative pain and increased joint range of motion. Group B had a lower incidence of pain during follow-up and showed significant functional improvement in Tip-pinch and Grip tests compared to Group A. U-shaped Kirschner wire fixation significantly reduced complications during the perioperative period. The U-shaped Kirschner wire internal fixation is a safe and effective treatment for the thumb proximal phalanx base ulnar side avulsion fracture.
Reference:
Ma, S., Zuo, J. & Hu, Y. U-shaped kirschner wire transfixation: effective treatment for Skier’s thumb. BMC Surg 24, 91 (2024). https://doi.org/10.1186/s12893-024-02382-7
Keywords:
U-shaped, Kirschner wire, internal fixation, safe and effective, treatment
Skier’s thumb, BMC Surgery, U-shaped Kirschner wire, Ulnar collateral ligament, Metacarpophalangeal joint, Bone fragmentation
Powered by WPeMatico