Erenumab: A Potential Breakthrough in Rosacea Management
Denmark: A nonrandomized controlled trial showed the importance of the calcitonin gene-related peptide (CGRP) pathway in the pathophysiology of erythema and flushing in rosacea. The findings suggested that calcitonin gene-related peptide receptor inhibition holds potential in treating rosacea-associated erythema and flushing.
“The study comprising 30 individuals with rosacea-associated flushing and erythema found that subcutaneous injections of monoclonal antibodies against the calcitonin gene-related peptide receptor administered every four weeks for 12 weeks significantly decreased days with erythema and flushing by weeks 9 through 12 compared with baseline,” the researchers reported. The findings were published online in JAMA Dermatology on April 17, 2024.
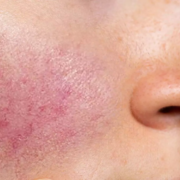
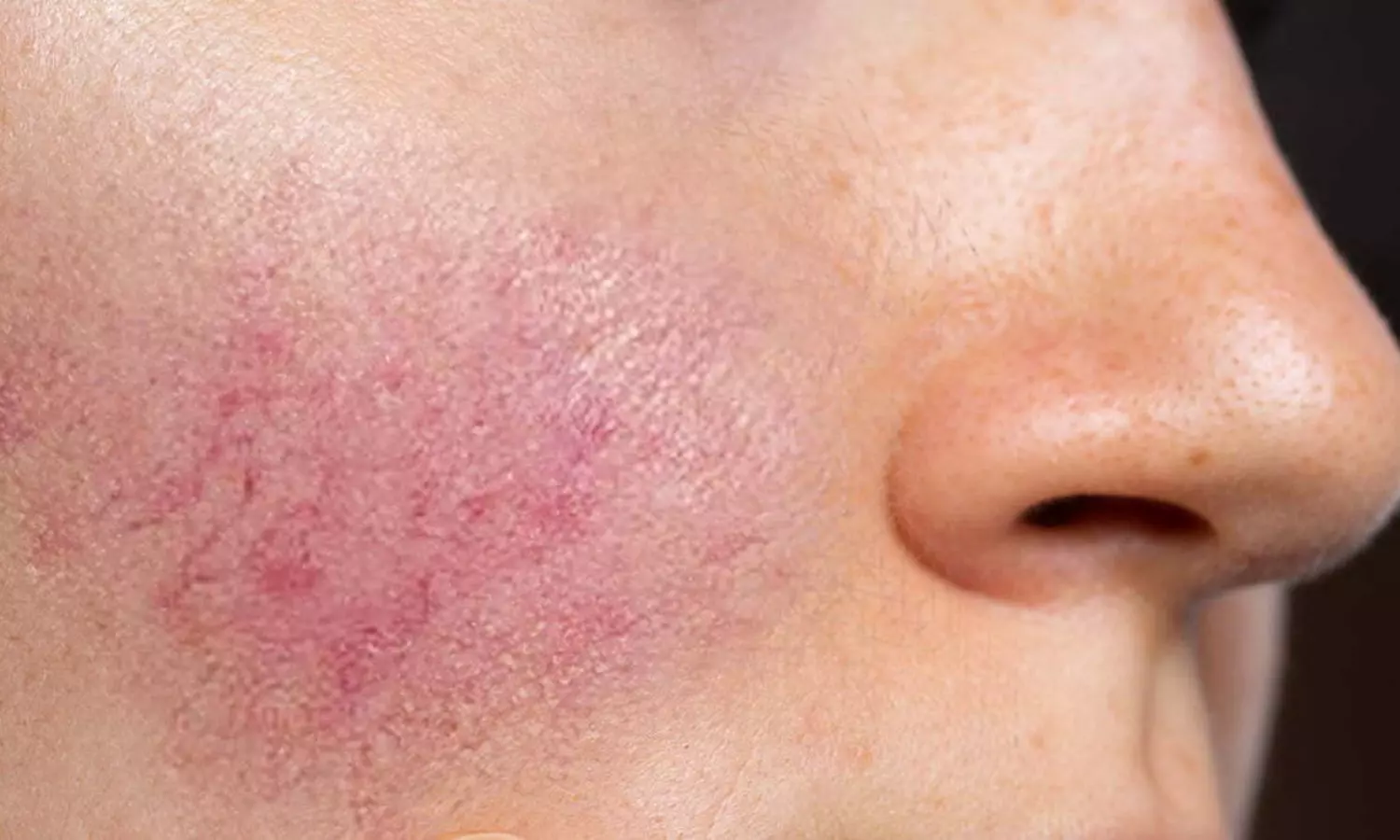
Rosacea is characterized by facial redness, visible blood vessels, and in some cases, papules and pustules. Persistent erythema and flushing are hallmark features, often resistant to conventional therapies. This affects patients’ physical appearance and psychological well-being, highlighting the need for effective treatment options.
While several treatment modalities exist, managing these symptoms remains a challenge. However, recent research suggests a promising avenue with erenumab use, a monoclonal antibody targeting CGRP. Nita K. F. Wienholtz, Copenhagen University Hospital-Rigshospitalet, Copenhagen, Denmark, and colleagues aimed to examine the tolerability, effectiveness, and safety of erenumab, an anti–CGRP-receptor monoclonal antibody, for the treatment of rosacea-associated erythema and flushing.
For this purpose, the researchers conducted a single-center, open-label, single-group, nonrandomized controlled trial between 2020 and 2021. Eligible participants included patients with rosacea with at least 15 days of either moderate to severe erythema and/or moderate to extreme flushing. Throughout the study period, no concomitant rosacea treatment was allowed.
Participants received 140 mg of erenumab subcutaneously every four weeks for 12 weeks. At week 20, a safety follow-up was performed. A total of 30 participants (mean age, 38.8 years; 77% females were included, of whom 27 completed the 12-week study.
The primary outcome was a mean change in the number of days with moderate to extreme flushing during weeks 9 through 12, compared with the 4-week run-in period (baseline). The secondary outcome was a mean change in the number of days with moderate to severe erythema. Adverse events were recorded for patients who received at least one dose of erenumab.
Following were the study’s key findings:
- There was a reduction in the mean number of days with moderate to extreme flushing by −6.9 days from 23.6 days at baseline.
- There was a reduction in the mean number of days with moderate to severe erythema by −8.1 days from 15.2 days at baseline.
- Adverse events included transient mild to moderate constipation (33% of participants), transient worsening of flushing (13% of participants), bloating (10% of participants), and upper respiratory tract infections (10% of participants), consistent with previous data.
- One participant discontinued the study due to a severe adverse event (hospital admission due to gallstones deemed unrelated to the study), and 2 participants withdrew consent due to lack of time.
In conclusion, the findings suggest that erenumab might be effective in reducing rosacea-associated flushing and chronic erythema (participants generally tolerated the treatment well, which was consistent with previous data). The study also showed that CGRP-receptor inhibition holds potential in treating flushing and erythema associated with rosacea.
“Larger randomized clinical trials are needed to confirm this finding,” the researchers wrote.
Reference:
Wienholtz NKF, Christensen CE, Do TP, et al. Erenumab for Treatment of Persistent Erythema and Flushing in Rosacea: A Nonrandomized Controlled Trial. JAMA Dermatol. Published online April 17, 2024. doi:10.1001/jamadermatol.2024.0408
Powered by WPeMatico