COPD Patients Face Earlier Mortality and Higher Comorbidity Burden: Study
Researchers have found in a real-world observational retrospective cohort study that patients with COPD died at a younger age and experienced more comorbidities compared to matched controls without COPD.
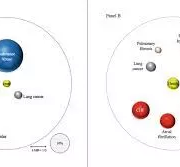
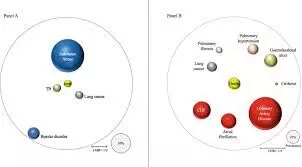
Patients with COPD suffer from various comorbidities, seemingly leading to a collective increase in morbidity and mortality. However, comorbidities with COPD have been largely unreported. Using healthcare claims data, only the deceased among around 250,000 COPD patients diagnosed in 2011– 2018 were evaluated by cause of death (cumulative incidence without competing risk) across a period of up to eight years. Results were compared with 1:1 propensity score-matched controls. Additionally, the prevalence of comorbidities in deceased patients was compared. Results: On average, deceased COPD patients and matched controls lived to be 75.7 and 78.0 years, respectively, and COPD patients had more comorbidities prior to death (mean 4.53 and 3.65). Both respiratory and cardiovascular-related deaths were more likely in COPD patients than in their matched controls (3.3 and 1.6 percentage points higher after eight years), and this was more extreme (9.8 and 3.4 percentage points higher, respectively) in the COPD subgroup with multiple/severe exacerbations; cumulative incidence of death increased with increasing COPD severity. Comorbidity prevalence, especially cardiovascular-related, was higher in COPD patients than in matched controls; COPD patients had a 42% higher risk of heart failure (RR 1.42; 1.38– 1.47), 30% higher risk of ischemic heart disease (RR 1.30; 1.25– 1.35), and 27% increased risk of atrial fibrillation (RR 1.27; 1.21– 1.32).
Conclusion: In this real-world observational retrospective cohort study, we found patients with COPD died at a younger age, and developed more comorbidities, than matched controls.
Reference:
Vogelmeier CF, Friedrich FW, Timpel P, Kossack N, Diesing J, Pignot M, Abram M, Gediga M, Halbach M. Comorbidities and Cause of Death in COPD Patients Compared to Non-COPD Controls: An 8-year Observational Retrospective Healthcare Claims Database Cohort Study. Int J Chron Obstruct Pulmon Dis. 2025;20:2117-2130
https://doi.org/10.2147/COPD.S488701
Keywords:
COPD, Patients, Face, Earlier, Mortality, Higher, Comorbidity, Burden, Study, exacerbations, mortality, cardiovascular disease, respiratory death, cardiovascular death, multimorbidity, Vogelmeier CF, Friedrich FW, Timpel P, Kossack N, Diesing J, Pignot M, Abram M, Gediga M, Halbach M. Comorbidities
Powered by WPeMatico