Hepatitis B is globally underassessed and undertreated, especially among women and Asian minorities in the West
Powered by WPeMatico
Powered by WPeMatico
Powered by WPeMatico
Powered by WPeMatico

Ridgefield: Boehringer Ingelheim has announced that the U.S. Food and Drug Administration (FDA) has approved the high-concentration, citrate-free formulation of Cyltezo (adalimumab-adbm), the company’s interchangeable* biosimilar to Humira (adalimumab), to treat multiple chronic inflammatory diseases.
“With this FDA approval, we are now able to offer both high- and low-concentration, citrate-free formulations of Cyltezo, further expanding treatment access for patients living with certain chronic inflammatory diseases,” said Stephen Pagnotta, Executive Director and Biosimilar Commercial Lead at Boehringer Ingelheim. “Many patients are treated with high-concentration adalimumab formulations, and we are excited to add this new option to our approved citrate-free Cyltezo and Adalimumab-adbm offerings.”
The high-concentration formulation (100 mg/mL), which is now available as a pre-filled syringe or pre-filled autoinjector, is priced at a 5% discount to Humira under the brand name Cyltezo and at an 81% discount to Humira as the unbranded product Adalimumab-adbm. The low-concentration (50 mg/mL), citrate-free formulation of Cyltezo has been commercially available since July 2023.
“The availability of a high-concentration and citrate-free Cyltezo is welcome news for people living with certain chronic inflammatory conditions, such as Crohn’s disease and ulcerative colitis, which affect nearly 1 in 100 Americans,” said Michael Osso, President & CEO of the Crohn’s & Colitis Foundation. “The flexibility of having multiple biosimilar formulations to choose from is important to support broader patient access to biologic medicines.”
“This FDA approval is another step forward for people with chronic and often debilitating diseases such as rheumatoid and psoriatic arthritis,” said Steven Taylor, President & CEO of the Arthritis Foundation. “We stand united with our patients and healthcare providers in the effort to accelerate the adoption of biosimilars, which benefit patients as well as the larger healthcare ecosystem.”
“Biologics and biosimilars are a crucial treatment option for many living with psoriatic arthritis or psoriasis,” said Leah M. Howard, J.D., President and CEO of the National Psoriasis Foundation. “We welcome the introduction of this additional formulation to expand the array of options available to our community.”
The FDA approval is based, in part, on data from clinical trial VOLTAIRE-HCLF, a Phase I clinical trial comparing the bioavailability of high-concentration and low-concentration formulations of adalimumab-adbm.
Powered by WPeMatico

New Delhi- The National Testing Agency (NTA) has finally released the admit cards for the candidates appearing in the National Eligibility cum Entrance Test- Under Graduate- NEET 2024.
Candidates can download the admit card from the official website of NTA using their application number and date of birth.
STEPS TO DOWNLOAD THE ADMIT CARD
STEP 1- Visit the Exam NTA official website.
STEP 2- Scroll down and open NEET-UG 2024 page.
STEP 3- Tap on “Click here for Admit Card” in the top left corner.
STEP 4- Enter application number, date of birth and security pin.
STEP 5- Click on the submit.
STEP 6- Admit Card will appear, check and download.
Candidates are urged to go through the instructions contained in the admit card as well as in the NEET-UG Information Bulletin, the official notice stated. However, the admit card contains information like the name of the applicant and centre, roll number and registration number, exam city, subjects with respective codes and reporting time.
If any candidate faces difficulty in downloading the admit card for NEET (UG) 2024, he/she can contact the number mentioned in the notice or email NTA, the notice added. Candidates are also advised to visit the official website of NTA and Exam NTA for the latest updates.
On Sunday, May 5, 2024, from 02:00 PM to 05:20 PM, NTA is going to conduct NEET-UG 2024 for more than 24 lakh candidates at various centres located in 557 cities across the country, including 14 cities outside India. The examination will be conducted in pen and paper mode. However, the result of NEET UG 2024 will be declared on June 14 2024.
Meanwhile, to facilitate the candidates appearing in NEET (UG)- 2024, advance information of the examination city where the examination centre will be located has already been shared by NTA.
To view the notice, click the link below
Powered by WPeMatico

Chennai: The medical community in the state is calling for stringent action
against misleading advertisements from Baba Ramdev’s Patanjali. At a recent state
conference in Trichy, healthcare professionals emphasized the need to regulate
unscientific medical information.
Dr G. R. Ravindranath, general secretary of the Doctor’s Association for Social Equality criticized
Ramdev for falsely claiming that Coronil could cure and prevent COVID-19. Dr Ravindranath accused Ramdev of publishing false advertisements and deceiving
the public with unverified medical claims.
Representatives of the Doctors Association for Social Equality argued
that Ramdev’s promotion of Ayurvedic medicines contradicts modern scientific
principles. They demanded strict measures against Ramdev for misleading people
under the pretence of promoting Ayurveda.
Citing Patanjali products as an example, the doctors advocate for a ban
on advertising pharmaceutical products without scientific evidence to support
their use. They insist that policies must be implemented to combat fake
science, superstitions, and anti-scientific notions in medicine, as these can
negatively affect individuals’ health and well-being across the country, reports DT Next.
The association stated, “We demand that the Union Government should apologize to act in favor of advertising such medicines. There should be stringent action against promoting medicines that can be harmful for the patients. The government needs to come up with policies on regulating mixopathy, Vedic medicine, traditional medicine, spiritual therapies and others, being practiced for treating diseases.”
Recently, a report in Reuters stated that a state regulator in India has suspended the manufacturing licenses of 14 products produced by pharmaceutical companies associated with the country’s renowned yoga guru. The suspension comes in response to the repeated dissemination of misleading advertisements regarding the efficacy of the products, as indicated by a government order.
The Supreme Court of India has in recent weeks repeatedly criticized Ramdev for not complying with its directives in an ongoing lawsuit to stop misleading advertisements of some of his traditional ayurvedic medicines. Though the company has already apologized for the unscientific advertisement, the Supreme Court asked whether the apology published by Patanjali Ayurved was as big as the advertisements.
Powered by WPeMatico

Srinagar: The Health and Medical Education Department in Jammu and
Kashmir made significant administrative changes on Tuesday, announcing new
appointment of Dr Rukhsana Jabeen as Principal Government Medical College (GMC) Anantnag and Dr Khurshid Wani as Principal Government Medical College (GMC) Handwara.
Dr Rukhsana has been serving as a
professor in the Department of Anesthesiology at Government Medical College
Srinagar. She takes over the role from Prof. Dr. Anjum Farhana, who was
previously serving as the interim principal at the Anantnag College. Additionally, Dr Khurshid Wani has been appointed as Principal of Government Medical College Handwara after Dr Iffat Hassan was relieved from the post of principal.
The order, issued on April 30, 2024, formalizes these
appointments and confirms the transitions. Both appointees bring years of
medical expertise and leadership to their new roles, aiming to continue and
improve the standards of medical education and patient care at their respective
institutions.
Powered by WPeMatico
Canada: In the realm of cardiovascular health, the coexistence of atrial fibrillation (AF) and heart failure (HF) presents a complex clinical challenge, necessitating nuanced treatment approaches. A recent systematic review and meta-analysis have shed light on the differential impact of atrial fibrillation ablation in patients with heart failure, stratified by ejection fraction status.
The study, published in JAMA Cardiology found that patients with heart failure with preserved ejection fraction (HFpEF) did not derive the same benefit from catheter ablation as patients with heart failure with reduced ejection fraction (HFrEF).
“The systematic review and meta-analysis of 12 randomized clinical trials (RCTs) comprising 2465 participants with HF, we found catheter ablation of AF compared with conventional medical therapies was tied to a reduced risk of HF events in patients with reduced ejection fraction, while no benefit was observed in patients with preserved ejection fraction,” the researchers reported.
Catheter ablation is associated with reduced heart failure hospitalization and death in select patients with atrial fibrillation (AF) and HFrEF. However, the benefit in patients with HFpEF is uncertain. Therefore, Alireza Oraii, Population Health Research Institute, Hamilton, Ontario, Canada, and colleagues aimed to investigate whether catheter ablation for AF is associated with reduced HF-related outcomes according to HF phenotype.
For this purpose, the researchers conducted a systematic search of online databases among studies published from inception to 2023.
The study included parallel-group RCTs comparing catheter ablation with conventional rate or rhythm control therapies in patients with HF, New York Heart Association (NYHA) functional class II or greater, and a history of paroxysmal or persistent AF. Pairs of independent reviewers screened 7531 titles and abstracts, of which the selection criteria were met by 12 RCTs and 4 substudies.
Data were abstracted in duplicate using Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guidelines.
The primary outcome was heart failure events, defined as HF hospitalization, clinically significant HF worsening, or unscheduled visits to a clinician for treatment intensification. Secondary outcomes were cardiovascular and all-cause mortality.
Following were the study’s key findings:
In conclusion, the study found that catheter ablation for AF was tied to a reduced risk of HF events in patients with HFrEF but had limited or no benefit in HFpEF.
“Results from ongoing trials may further elucidate the role of catheter ablation for atrial fibrillation in HFpEF,” the researchers wrote.
Reference:
Oraii A, McIntyre WF, Parkash R, et al. Atrial Fibrillation Ablation in Heart Failure With Reduced vs Preserved Ejection Fraction: A Systematic Review and Meta-Analysis. JAMA Cardiol. Published online April 24, 2024. doi:10.1001/jamacardio.2024.0675
Powered by WPeMatico
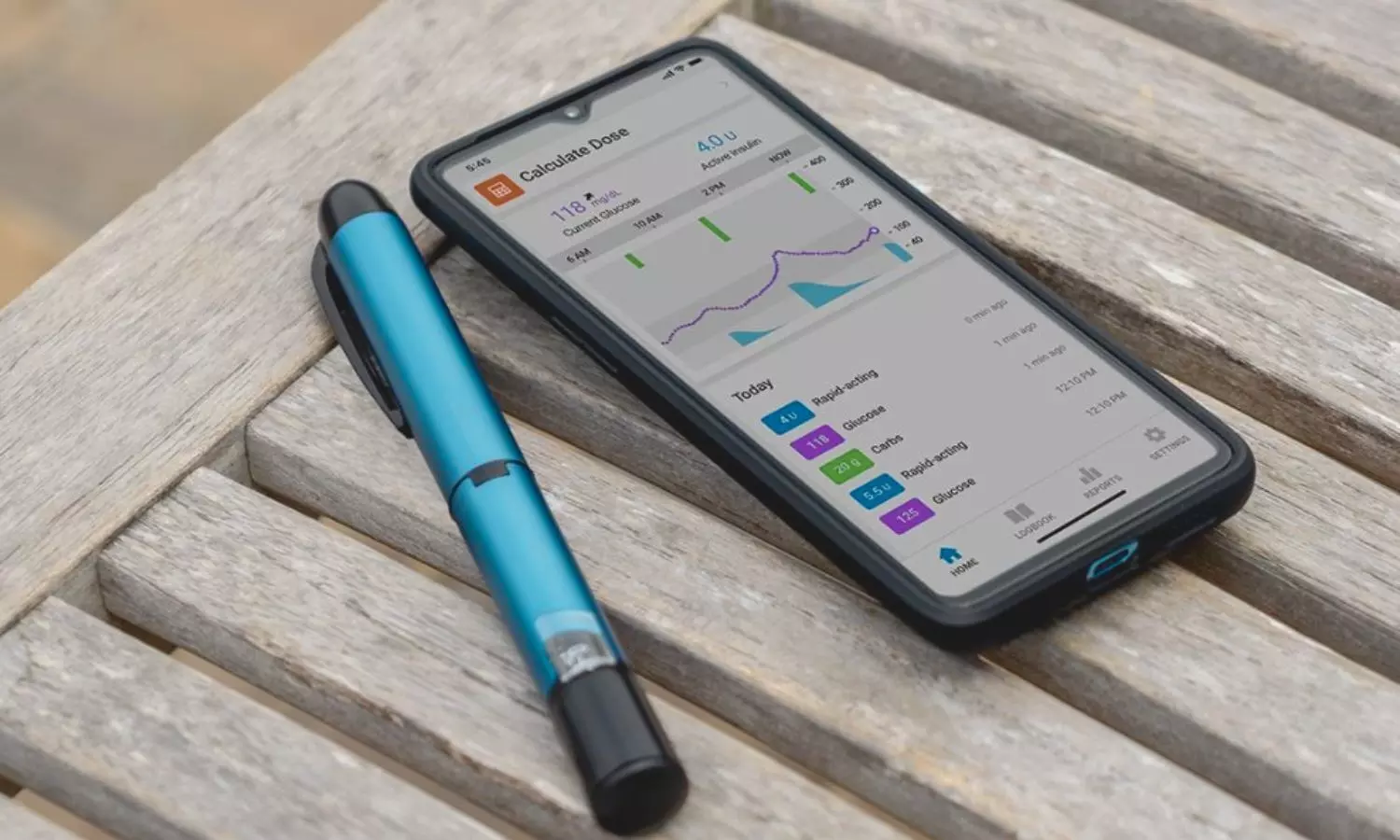
Germany: In the realm of diabetes management, the integration of technology has revolutionized treatment approaches, offering patients innovative solutions to monitor and control their condition better. A recent study investigating the association between treatment adherence and continuous glucose monitoring (CGM) outcomes among individuals with diabetes utilizing smart insulin pens in real-world settings has unveiled promising insights into the efficacy of these devices.
The combined analysis of real-world smart pen and CGM data revealed that missing two basal or four bolus insulin doses over 14 days would be tied with a clinically relevant reduction in time in range (TIR). Smart insulin pens provide valuable insights into treatment injection behaviors.
“Adults with diabetes receiving basal and bolus insulin through a smart pen miss about six doses every 14 days,” the researchers reported in Diabetes Care. Each missed basal or bolus insulin dose is tied to a reduction in time in range.”
Thomas Danne, Hanover Medical School, Hanover, Germany, and colleagues aimed to evaluate the association of insulin injection adherence, smart insulin pen engagement, and glycemic control using real-world data from 16 countries from adults self-administering basal insulin degludec and bolus insulin with a smart insulin pen (NovoPen 6 or NovoPen Echo Plus) alongside CGM.
For this purpose, data aggregation was done over 14-day periods. Treatment adherence was defined according to the number of missed basal and missed bolus insulin doses and smart pen engagement as per the number of days with data uploads.
The researchers analyzed data from 3,945 adults, including 25,157 14-day periods with ≥70% CGM coverage.
The researchers reported the following findings:
The integration of smart insulin pens into routine diabetes management regimens holds promise for optimizing treatment adherence and ultimately improving glycemic control. As technology continues to evolve, studies such as these contribute valuable insights that pave the way for advancements in diabetes care, offering hope for a future where individuals can better manage their condition and enjoy improved quality of life.
Reference:
Thomas Danne, Michael Joubert, Niels Væver Hartvig, Anne Kaas, Nikoline Nygård Knudsen, Julia K. Mader; Association Between Treatment Adherence and Continuous Glucose Monitoring Outcomes in People With Diabetes Using Smart Insulin Pens in a Real-World Setting. Diabetes Care 2024; dc232176. https://doi.org/10.2337/dc23-2176
Powered by WPeMatico
In Europe and North America, 30 to 60% of dietary energy intake in adults comes from ultra-processed foods. An increasing number of epidemiological studies suggest a link between higher consumption levels of ultra-processed foods with higher risks of diabetes and other metabolic disorders.
Emulsifiers are among the most commonly used additives. They are often added to processed and packaged foods such as certain industrial cakes, biscuits and desserts, as well as yoghurts, ice creams, chocolate bars, industrial breads, margarines and ready-to-eat or ready-to-heat meals, in order to improve their appearance, taste and texture and lengthen shelf life. These emulsifiers include for instance mono- and diglycerides of fatty acids, carrageenans, modified starches, lecithins, phosphates, celluloses, gums and pectins.
As with all food additives, the safety of emulsifiers had been previously evaluated by food safety and health agencies based on the scientific evidence that was available at the time of their evaluation. However, some recent studies suggest that emulsifiers may disrupt the gut microbiota and increase the risk of inflammation and metabolic disruption, potentially leading to insulin resistance and the development of diabetes.
For the first time worldwide, a team of researchers in France has studied the relationships between the dietary intakes of emulsifiers, assessed over a follow-up period of maximum 14 years, and the risk of developing type 2 diabetes in a large study in the general population.
The results are based on the analysis of data from 104 139 adults in France (average age 43 years; 79% women) who participated in the NutriNet-Santé web-cohort study (see box below) between 2009 and 2023.
The participants completed at least two days of dietary records, collecting detailed information on all foods and drinks consumed and their commercial brands (in the case of industrial products). These dietary records were repeated every six months for 14 years, and were matched against databases in order to identify the presence and amount of food additives (including emulsifiers) in the products consumed. Laboratory assays were also performed in order to provide quantitative data. This allowed a measurement of chronic exposure to these emulsifiers over time.
During follow-up, participants reported the development of diabetes (1056 cases diagnosed), and reports were validated using a multi-source strategy (including data on diabetes medication use). Several well-known risk factors for diabetes, including age, sex, weight (BMI), educational level, family history, smoking, alcohol and levels of physical activity, as well as the overall nutritional quality of the diet (including sugar intake) were taken into account in the analysis.
After an average follow-up of seven years, the researchers observed that chronic exposure – evaluated by repeated data – to the following emulsifiers was associated with an increased risk of type 2 diabetes:
This study constitutes an initial exploration of these relationships, and further investigations are now needed to establish causal links. The researchers mentioned several limitations of their study, such as the predominance of women in the sample, a higher level of education than the general population, and generally more health-promoting behaviours among the NutriNet-Santé study participants. Therefore caution is needed when extrapolating the conclusions to the entire French population.
The study is nevertheless based on a large sample size, and the researchers have accounted for a large number of factors that could have led to confounding bias. They also used unique, detailed data on exposure to food additives, down to the commercial brand name of the industrial products consumed. In addition, the results remain consistent through various sensitivity analyses, which reinforces their reliability.
‘These findings are issued from a single observational study for the moment, and cannot be used on their own to establish a causal relationship. They need to be replicated in other epidemiological studies worldwide, and supplemented with toxicological and interventional experimental studies, to further inform the mechanisms linking these food additive emulsifiers and the onset of type 2 diabetes. However, our results represent key elements to enrich the debate on re-evaluating the regulations around the use of additives in the food industry, in order to better protect consumers,’ explain Mathilde Touvier, Research Director at Inserm, and Bernard Srour, Junior Professor at INRAE, lead authors of the study.
Among the next steps, the research team will be looking at variations in certain blood markers and the gut microbiota linked to the consumption of these additives, to better understand the underlying mechanisms. The researchers will also look at the health impact of additive mixtures and their potential ‘cocktail effects.’ They will also work in collaboration with toxicologists to test the impact of these exposures in in vitro and in vivo experiments, to gather more arguments in favour of a causal link.
Reference:
Clara Salame, Guillaume Javaux, Laury Sellem, Emilie Viennois, Fabien Szabo de Edelenyi, Cédric Agaësse, Food additive emulsifiers and the risk of type 2 diabetes: analysis of data from the NutriNet-Santé prospective cohort study, The Lancet Diabetes & Endocrinology, https://doi.org/10.1016/S2213-8587(24)00086-X.
Powered by WPeMatico
