SSRIs in Pregnancy may Affect Maternal Care giving behavior, Not Offspring Cognition, reveals study
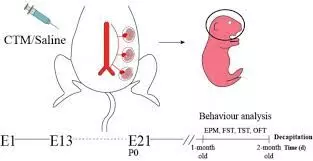
Findings from a new study show that selective serotonin reuptake inhibitor use during pregnancy and lactation may alter maternal caregiving behaviors but does not impair offspring cognition. Selective serotonin reuptake inhibitors, such as fluoxetine, are widely prescribed for the treatment of depression and anxiety in women of reproductive age, including during pregnancy. Concerns about potential risks for neurodevelopmental impairment in children have often complicated treatment decisions, leading to hesitation among patients and clinicians.
The study offers important clarification by indicating that while maternal behaviors related to caregiving may be influenced by exposure to selective serotonin reuptake inhibitors during the perinatal period, offspring cognitive abilities remain unaffected. Maternal caregiving encompasses essential interactions such as attentiveness, emotional responsiveness, and nurturing practices, all of which are critical for healthy child development.
Pharmacological modulation of serotonin systems during pregnancy may influence these behaviors, underscoring the importance of monitoring and supporting maternal-infant bonding in women undergoing pharmacological treatment. At the same time, the absence of measurable adverse effects on offspring learning, memory, and cognitive performance provides reassurance regarding long-term neurodevelopmental outcomes.
This distinction helps shift the clinical conversation from concerns about irreversible cognitive harm in children to a more practical focus on supporting maternal caregiving capacity. Untreated maternal depression itself carries significant risks, including reduced caregiving quality and poorer child outcomes, highlighting the necessity of balancing risks and benefits when considering treatment options. The findings encourage clinicians to take an integrative approach, combining pharmacological management with psychosocial support strategies that reinforce maternal caregiving behaviors. Further longitudinal research will be important to understand whether early behavioral changes in mothers translate into long-term psychosocial impacts on offspring.
Overall, the evidence suggests that selective serotonin reuptake inhibitors remain a viable therapeutic option during pregnancy, provided maternal support systems are emphasized as part of comprehensive perinatal care.
Keywords: selective serotonin reuptake inhibitors, fluoxetine, pregnancy, lactation, maternal caregiving, offspring cognition, perinatal depression, child development
Powered by WPeMatico