How small changes in walking technique may help treat knee osteoarthritis
Powered by WPeMatico
Powered by WPeMatico
Powered by WPeMatico
Powered by WPeMatico
Powered by WPeMatico

India: A large multicentre clinical trial conducted in India has found that three commonly prescribed dual-drug regimens for high blood pressure are equally effective and well-tolerated in South Asian adults. The findings, published in Nature Medicine, provide important evidence to guide hypertension management in the region, where cardiovascular disease remains a major cause of illness and death.
Led by Dorairaj Prabhakaran and colleagues from the Centre for Chronic Disease Control in New Delhi, the study aimed to address a significant evidence gap in optimising combination therapy for South Asian patients. While dual therapy is often recommended when single medications fail to achieve adequate blood pressure control, there has been little comparative data on which combinations work best in this population.
The trial enrolled 1,981 Indian adults aged 30 to 79 years, with a mean age of 52 years. Participants had either a sitting systolic blood pressure (SBP) of 150–179 mmHg while untreated, or an SBP of 140–159 mmHg while already on a single medication. They were randomly assigned, in equal proportions, to receive one of three single-pill combinations: amlodipine–perindopril, perindopril–indapamide, or amlodipine–indapamide. The primary goal was to measure changes in 24-hour ambulatory SBP after six months of treatment.
The study led to the following findings:
The trial, known as the TOPSPIN trial, is one of the largest head-to-head comparisons of dual therapy in Indian patients. According to the authors, the results suggest that any of these combinations—amlodipine–perindopril, perindopril–indapamide, or amlodipine–indapamide—can be considered equally valid first choices for initiating dual therapy in South Asian adults with hypertension.
Given that elevated blood pressure is a leading risk factor for heart attacks, strokes, and kidney disease, the researchers believe the findings will help clinicians tailor treatment strategies without being restricted to one specific combination. The comparable efficacy and safety profiles of these regimens may also allow flexibility based on patient preferences, cost, and availability.
By providing robust, region-specific evidence, the study addresses a critical gap in hypertension management for South Asians, both in India and across the global diaspora, potentially improving blood pressure control and reducing cardiovascular disease burden.
Reference:
Prabhakaran, D., Roy, A., Chandrasekaran, A. M., Kondal, D., Mukherjee, S., Kiru, G., Singh, K., Salwa, H., Sobitharaj, E. C., Lobo, A. S., Mahajan, G., Mohan, B., Khanna, A., Malviya, A., Patil, S. G., Abichandani, V. K., Singh, B., Gupta, B. K., Yellapantula, B., . . . Poulter, N. R. (2025). Comparison of dual therapies for hypertension treatment in India: A randomized clinical trial. Nature Medicine, 1-7. https://doi.org/10.1038/s41591-025-03854-w
Powered by WPeMatico
Powered by WPeMatico
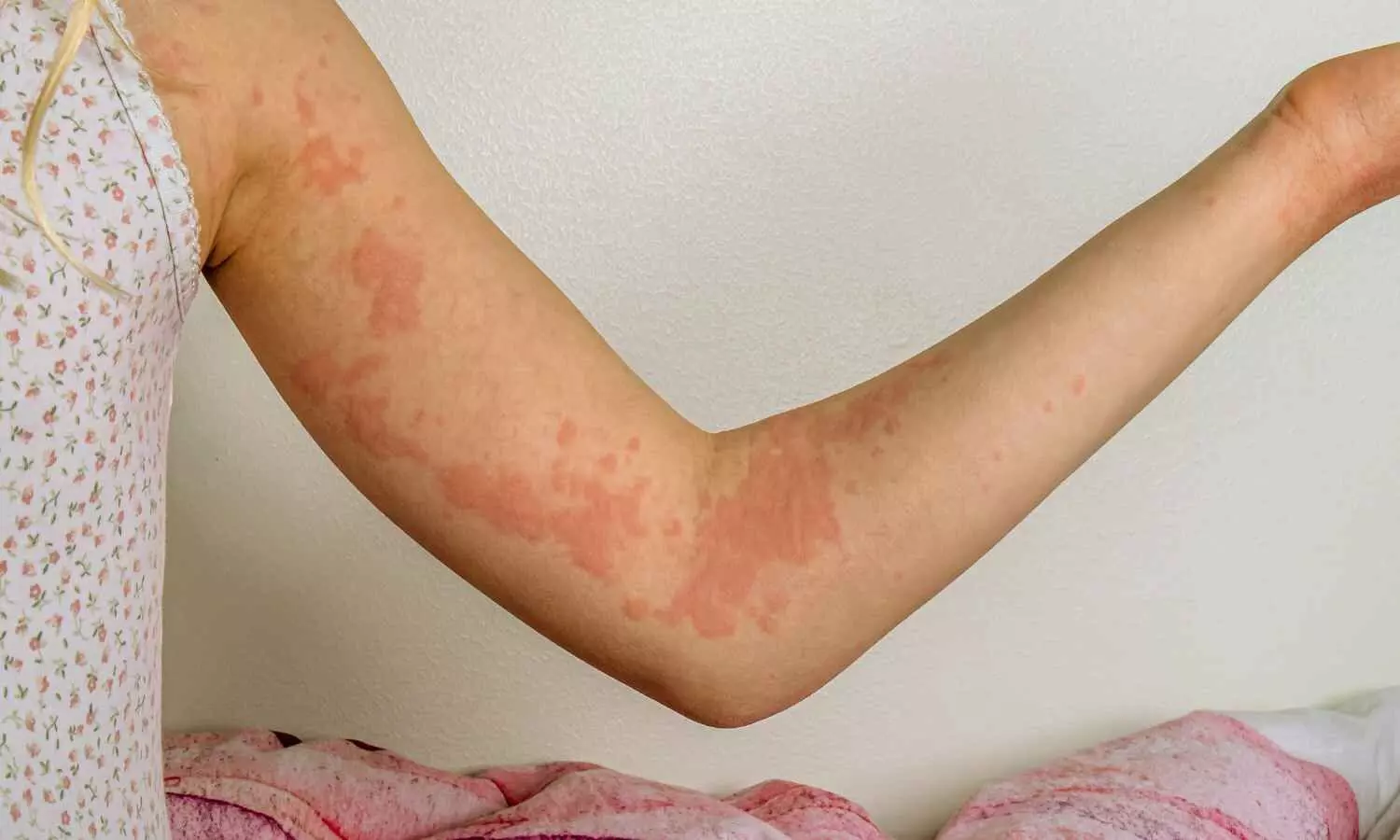
A new study published in the Journal of American Medical Association found that Rilzabrutinib decreased itching and hives while preserving a positive risk-benefit profile, indicating that it might be a useful therapy for individuals with moderate to severe chronic spontaneous urticaria (CSU) that is resistant to antihistamines.
The primary cause of CSU is the activation of cutaneous mast cells via a variety of pathways. B cells and mast cells contain the protein bruton tyrosine kinase (BTK), which is essential for several immune-mediated disease processes. Thus, this research evaluated the effectiveness and risk profile of rilzabrutinib, a covalent, oral, reversible, next-generation BTK inhibitor, in the treatment of patients with CSU.
The Rilzabrutinib Efficacy and Safety in CSU (RILECSU) randomized clinical trial was a 52-week phase 2 research that included a 40-week open-label extension after a 12-week double-blind, dose-ranging, placebo-controlled period. From November 24, 2021, until April 23, 2024, the trial was held.
12 countries across Asia, Europe, North America, and South America, 51 centers recruited and randomly assigned participants. Adults with moderate to severe CSU (weekly Urticaria Activity Score [UAS7] of 16 or more; weekly Itch Severity Score [ISS7] of 8 or higher) who were not well managed with H1-antihistamine medication were enrolled in the experiment. This ranged in age from 18 to 80 and the patients were randomized 1:1:1:1 to 400 mg of rilzabrutinib, 400 mg once a day in the evening, 800 mg twice daily, 1200 mg three times daily, or a matched placebo.
160 omalizumab-naive and omalizumab-incomplete responders (mean [SD] age, 44.1 [13.4] years; 112 [70.0%] females) were randomly assigned. Only the 143 individuals who had never used omalizumab were part of the primary analysis population. ISS7 (least squares [LS] mean, −9.21 vs −5.77; difference, −3.44 [95% CI, −6.25 to −0.62]; P =.02) and UAS7 (LS mean, −16.89 vs −10.14; difference, −6.75 [95% CI, −12.23 to −1.26) showed significant decreases at week 12 when rilzabrutinib, 1200 mg/d, was compared to placebo from baseline.
Improvements were also seen in the weekly Angioedema Activity Score (AAS7) and weekly Hives Severity Score (HSS7). As early as week 1, ISS7, UAS7, HSS7, and AAS7 showed improvements. At week 12, CSU-related biomarkers, such as immunoglobulin (Ig)-G antithyroid peroxidase, soluble Mas-related G protein–coupled receptor X2, IgG anti-Fc-ε receptor 1, and interleukin-31, were lower than placebo.
Overall, along with an acceptable adverse event profile, the RILECSU randomized clinical trial findings showed that rilzabrutinib, 1200 mg/d, was effective and had a quick start of action over a 12-week period.
Reference:
Giménez-Arnau, A., Ferrucci, S., Ben-Shoshan, M., Mikol, V., Lucats, L., Sun, I., Mannent, L., & Gereige, J. (2025). Rilzabrutinib in antihistamine-refractory chronic spontaneous urticaria: The RILECSU phase 2 randomized clinical trial: The RILECSU phase 2 randomized clinical trial. JAMA Dermatology (Chicago, Ill.), 161(7), 679–687. https://doi.org/10.1001/jamadermatol.2025.0733
Powered by WPeMatico

USA: A recent large-scale investigation published in the Journal of Periodontology by Muhammad H. A. Saleh and Hamoun Sabri from the University of Michigan School of Dentistry has revealed a dose-dependent relationship between the severity of periodontitis and the presence of multiple systemic health conditions.
Using data from electronic health records spanning 2013 to 2023, the researchers analyzed 264,913 adult patients treated at nine U.S. dental schools, examining the links between gum disease severity—classified as none, mild/moderate, or severe—and 24 selected systemic and behavioral conditions.
The study revealed the following notable findings:
The findings highlight the complex interplay between oral and systemic health, suggesting that worsening gum disease may reflect or exacerbate underlying medical conditions. While the research did not establish cause-and-effect—owing to its cross-sectional design—it highlights the value of periodontal assessment as a potential marker for broader health risks. The study emphasizes that both medical and dental professionals should collaborate more closely in managing patients with chronic conditions to ensure that oral health is integrated into overall healthcare strategies.
The scale of the dataset, covering over a quarter of a million patients, lends robustness to the results; however, the authors caution about certain limitations. The reliance on treatment codes as indicators of disease severity may not fully capture clinical nuances. Additionally, because the data came from individuals actively receiving dental care, the sample might not perfectly represent the general population. Other potential confounding factors, such as specific oral hygiene habits, were not available, leaving the possibility of residual bias.
Despite these caveats, the study’s implications are significant. The clear gradient of risk—where odds ratios for severe periodontitis consistently exceeded those for milder disease—suggests that monitoring gum health could help identify individuals at greater risk for conditions like diabetes, cardiovascular disease, HIV, and Alzheimer’s. Conversely, protective patterns with certain illnesses, such as asthma, point to areas where further research is needed.
“Overall, the work reinforces the message that oral health is inseparable from systemic health and should be prioritized in comprehensive patient care,” the authors concluded.
Reference:
A. Saleh, M. H., & Sabri, H. Dose-dependent association of systemic comorbidities with periodontitis severity: A large population cross-sectional study. Journal of Periodontology. https://doi.org/10.1002/JPER.25-0055
Powered by WPeMatico
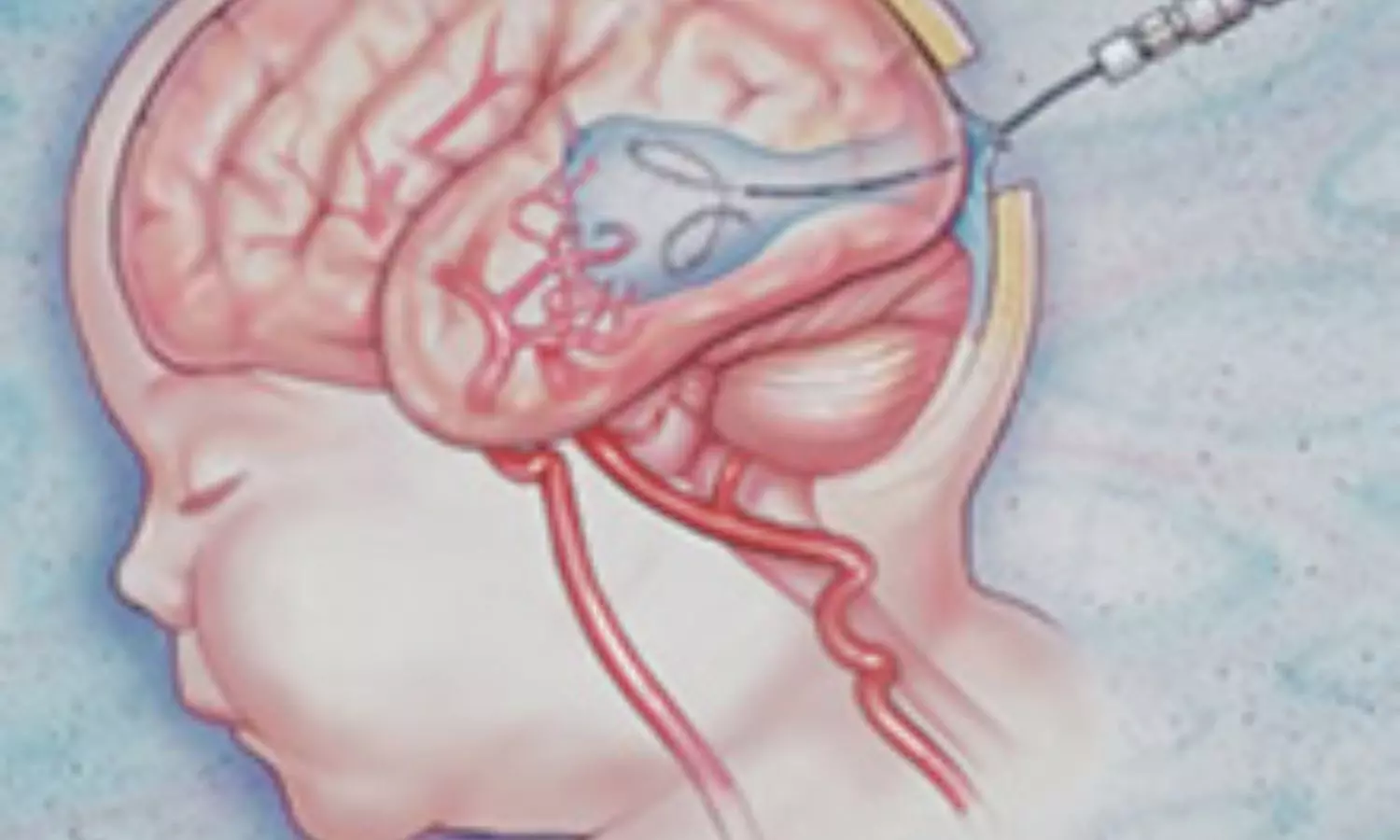
USA: Early single-center data suggest that in utero embolization for high-risk Vein of Galen Malformation (VOGM) is feasible, with surviving infants showing normal neurodevelopment; however, the risk of preterm delivery needs further investigation.
A preliminary communication published in JAMA by Dr. Darren B. Orbach and colleagues from Boston Children’s Hospital describes the first systematic attempt to treat this rare congenital cerebrovascular condition before birth. VOGM, the most common fetal brain blood vessel malformation, can cause life-threatening complications after delivery, particularly when the mediolateral falcine sinus is significantly widened. Infants with high-risk measurements face extremely poor survival odds and a high likelihood of neurodevelopmental delay under standard postnatal care.
The study, conducted at a single U.S. center between September 2022 and April 2025, enrolled seven fetuses diagnosed with high-risk VOGM. Eligibility required no major brain injury on fetal MRI and a falcine sinus width of at least 7 mm. Using ultrasound guidance, the team accessed the fetal brain through the uterus and skull, navigating a microcatheter to the malformed vein and deploying detachable coils to reduce abnormal blood flow.
The following were the key findings of the study:
The authors note that historically, fetuses with similar severity measurements have a mortality risk approaching 90%, with only about 9% achieving early developmental milestones under standard treatment. In the current study, overall mortality was 43%, and 43% of infants were on track at six months.
While the results are encouraging, the researchers stress that the findings are preliminary. The small sample size reflects the rarity of high-risk fetal VOGM, and the procedures were all performed in a high-volume tertiary care setting by specialists with significant experience in fetal and neurointerventional surgery. Longer-term follow-up will be essential to fully assess developmental outcomes, and broader multicenter studies will be required to determine if the approach can be replicated safely elsewhere.
“Still, this represents a landmark in fetal neurosurgery. As the research team notes, it is the first targeted effort to treat a congenital cerebrovascular anomaly before birth by directly altering fetal brain blood flow. The approach, while promising, must be weighed carefully against the increased risk of preterm delivery, with decisions guided by multidisciplinary expertise,” the authors concluded.
Reference:
Orbach DB, Shamshirsaz AA, Wilkins-Haug L, et al. In Utero Embolization for Fetal Vein of Galen Malformation. JAMA. Published online August 11, 2025. doi:10.1001/jama.2025.12363
Powered by WPeMatico
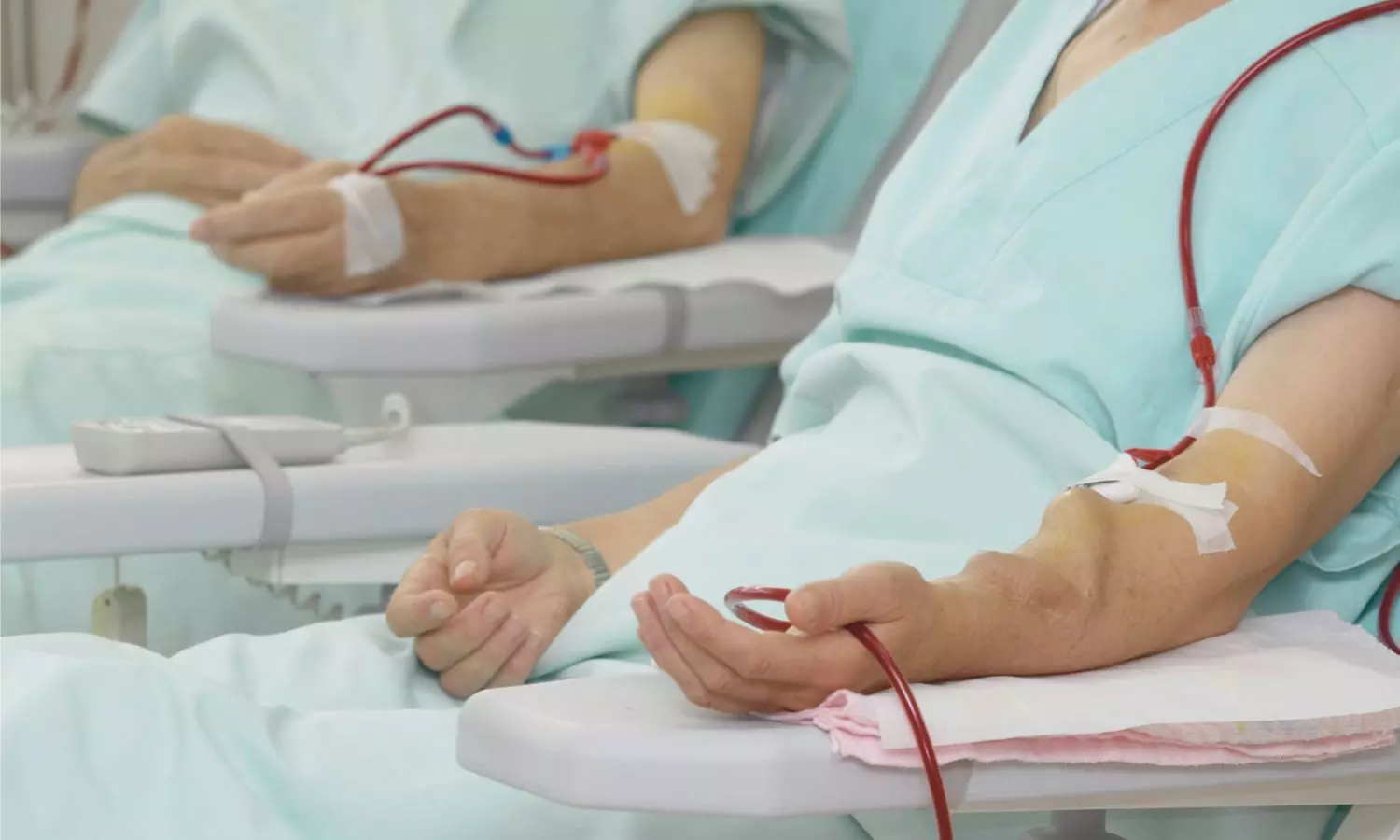
Sweden: In a significant advancement for dialysis care, researchers from Linkoping University, Sweden, have found that chlorhexidine is more effective than ethanol in reducing bacterial presence during buttonhole cannulation in patients undergoing haemodialysis via arteriovenous fistula (AVF). The study, led by Karin Staaf and colleagues from the Department of Health, Medicine and Caring Sciences, was published in BMC Nephrology.
AVF infections are commonly caused by the patient’s own skin bacteria, and the buttonhole technique—where needles are inserted at the same site each time—increases this risk due to repeated skin penetration. While proper disinfection is essential to mitigate this risk, there has been limited evidence guiding the optimal disinfectant choice. This study aimed to determine whether chlorhexidine offers superior protection compared to ethanol in this setting.
The randomized, crossover trial involved patients undergoing haemodialysis, comparing 5 mg/mL chlorhexidine in 70% ethanol against 70% ethanol alone, both with and without prior arm washing. Bacterial samples were collected at multiple time points: before disinfection, immediately after, and two and four hours post-disinfection, across four dialysis sessions. Additionally, scabs from the buttonhole tract were analyzed to identify the types of bacteria present and whether they matched the patient’s normal skin flora.
The key findings were as follows:
Though the study demonstrated promising results, the researchers acknowledged several limitations. The use of colony-forming units (CFU/mL) as a surrogate marker rather than actual infection rates was necessary due to the ethical and logistical challenges of a large-scale infection trial. The single-centre nature of the study, its small sample size, and the fact that it was only single-blinded may also influence generalizability. Furthermore, patient behavior during dialysis—such as using the disinfected arm for support or covering it—may have introduced variability in outcomes.
Despite these constraints, the research provides valuable insight into infection control practices in dialysis units. The use of chlorhexidine not only delays bacterial regrowth but also potentially reduces the overall risk of AVF-related infections. As the authors emphasized, preventing infections caused by patients’ own skin flora is a critical responsibility for dialysis providers, and adopting chlorhexidine-based disinfection protocols could be a step forward in achieving this goal.
The authors concluded, “The study adds to the growing body of evidence suggesting that chlorhexidine may be a more reliable option than ethanol for disinfection in buttonhole cannulation, thereby enhancing patient safety in haemodialysis settings.”
Reference:
Staaf, K., Scheer, V., Serrander, L. et al. Disinfection with chlorhexidine is more effective than ethanol for buttonhole cannulation in arteriovenous fistula: a randomized cross-over trial. BMC Nephrol 26, 402 (2025). https://doi.org/10.1186/s12882-025-04230-z
Powered by WPeMatico
