ADMIRE Trial Confirms Safety and Effectiveness of Pulsed Field Ablation for Paroxysmal AF
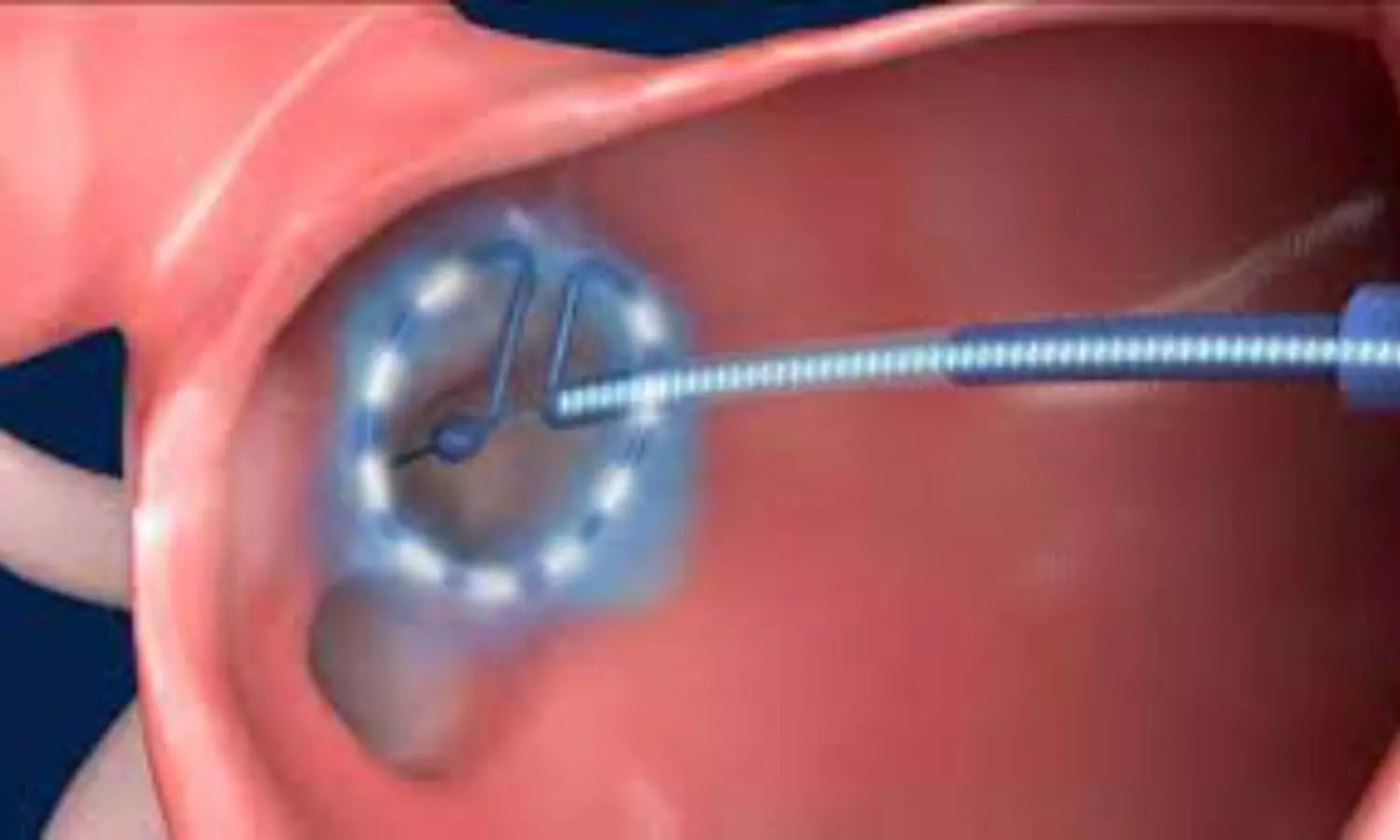
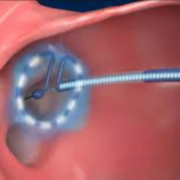
USA: The ADMIRE pivotal trial has provided significant insights into using pulsed-field ablation (PFA) for treating paroxysmal atrial fibrillation, demonstrating safety and effectiveness. This innovative approach utilizes a variable-loop PFA catheter, which facilitates shorter procedure times and reduces exposure to fluoroscopy.
“The ADMIRE trial confirmed that the variable-loop PFA catheter is both safe and effective, demonstrating short procedure and PFA application times, along with low levels of fluoroscopy exposure,” the researchers wrote in Circulation.
Atrial fibrillation (AF) is a common cardiac arrhythmia that can lead to severe complications, including stroke and heart failure. Paroxysmal atrial fibrillation, characterized by intermittent episodes, is particularly challenging to manage. Traditional ablation methods have their drawbacks, including longer recovery times and higher radiation exposure for patients and medical staff.
Clinical trials of early PFA systems for treating atrial fibrillation have shown promising potential to minimize complications associated with traditional thermal methods while preserving efficacy. However, the absence of a fully integrated mapping system—commonly used in contemporary electrophysiology procedures—limits lesion creation and workflow efficiency. To address this, a novel variable-loop PFA catheter has been developed, which integrates with an electroanatomic mapping system. This advancement enables real-time nonfluoroscopic procedural guidance, lesion indexing, and feedback on the proximity between tissue and catheter.
In light of this context, Vivek Y. Reddy and colleagues from the Helmsley Electrophysiology Center at Mount Sinai Fuster Heart Hospital in New York, NY, conducted the ADMIRE trial. This multicenter, single-arm study, which received an investigational device exemption from the Food and Drug Administration, evaluated the long-term safety and effectiveness of the integrated pulsed field ablation (PFA) system in a large population of drug-refractory patients with symptomatic paroxysmal atrial fibrillation in the United States.
Using the PFA catheter in conjunction with a compatible electroanatomic mapping system, patients with drug-refractory symptomatic paroxysmal atrial fibrillation underwent pulmonary vein isolation.
The primary safety endpoint was the occurrence of major adverse events within seven days post-ablation. The primary effectiveness endpoint was a composite measure that included 12 months of freedom from documented episodes of atrial tachyarrhythmia (such as atrial fibrillation, atrial tachycardia, and atrial flutter), failure to achieve pulmonary vein isolation, use of a non-study catheter for isolation, repeat procedures (excluding one redo during the blanking period), initiation of a new or previously ineffective class I or III antiarrhythmic drug at a higher dose after the blanking period, or the need for direct current cardioversion after the blanking phase.
Based on the study, the researchers reported the following findings:
- At 30 centers, 277 patients with paroxysmal atrial fibrillation (61.5±10.3 years of age; 64.3% male) in the pivotal cohort underwent PFA.
- More than 25% of the procedures were performed without fluoroscopy. Median pulmonary vein isolation procedure, fluoroscopy, and transpired PFA application times were 81.0, 7.1, and 31.0 minutes, respectively.
- The primary adverse event rate was 2.9%, with the most common complication being pericardial tamponade.
- The 12-month primary effectiveness endpoint was 74.6%.
- The 1-year freedom from atrial fibrillation, atrial tachycardia, or atrial flutter recurrence rate after blanking was 75.4%.
- There were substantial improvements in quality of life as early as three months after the procedure, concurrent with a reduction in multiple healthcare use measures.
In conclusion, the ADMIRE pivotal trial confirms that pulsed field ablation with the variable-loop catheter is a promising treatment option for patients with paroxysmal atrial fibrillation.
“With shorter procedure times and reduced fluoroscopy exposure, this innovative technique could significantly improve patient outcomes and quality of life for those affected by this common cardiac condition,” the researchers wrote.
Reference:
Reddy VY, Calkins H, Mansour M, et al. Pulsed-field ablation to treat paroxysmal atrial fibrillation: safety and effectiveness in the ADMIRE pivotal trial. Circulation. 2024; Epub ahead of print.
Powered by WPeMatico