Telitacicept Improves Clinical Response but Increases Infection Risk in Active SLE: NEJM
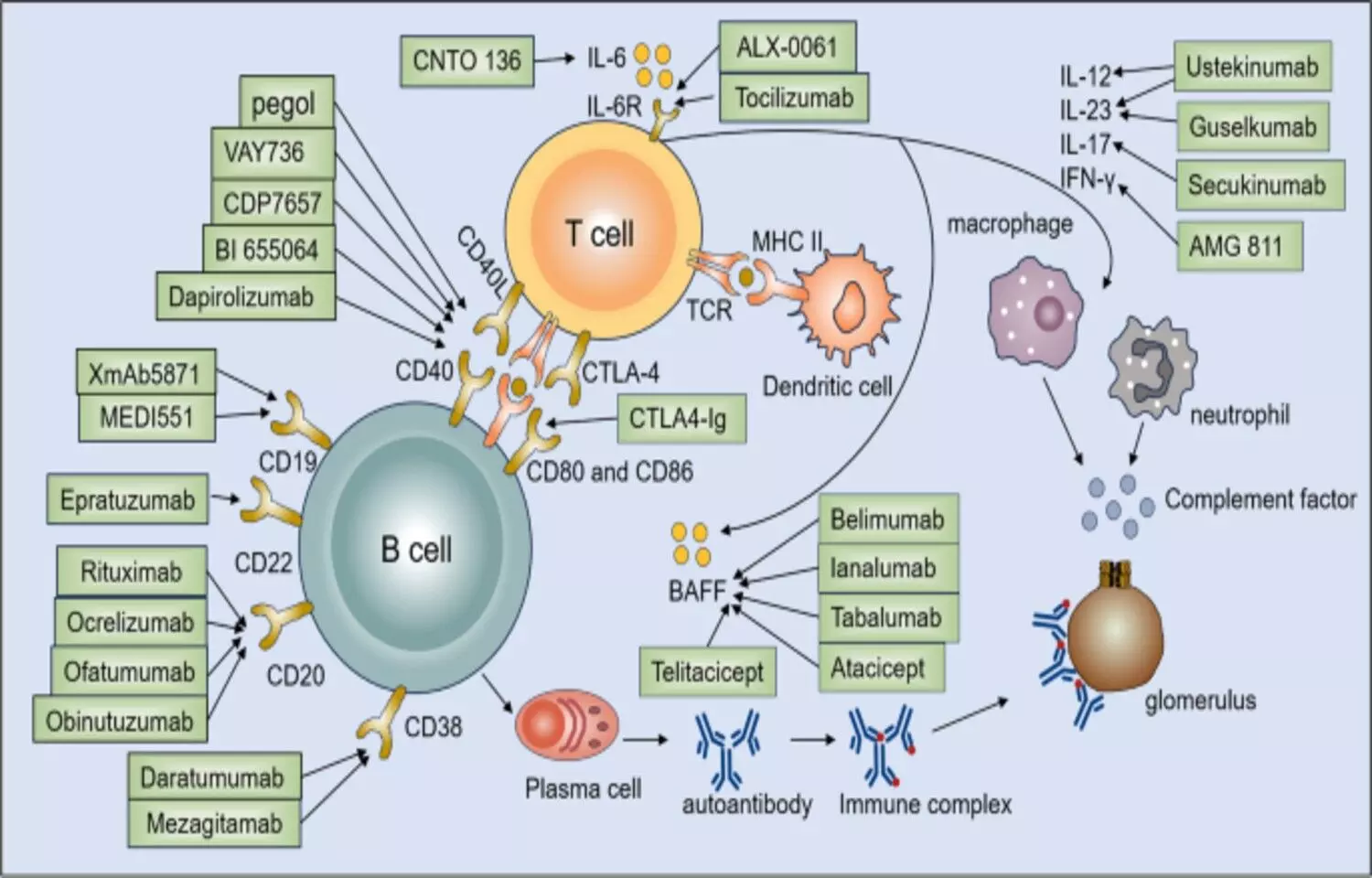
In a 52-week trial of patients with active systemic lupus erythematosus (SLE) receiving standard background therapy, telitacicept produced a higher clinical response rate compared with placebo. The study, published in the New England Journal of Medicine, evaluated the efficacy and safety of telitacicept, a dual B-cell activating factor (BAFF) and APRIL inhibitor. Patients treated with telitacicept achieved significantly greater improvements in disease activity scores, reflecting better control of lupus manifestations such as rash, arthritis, and serologic activity. However, the treatment was also linked to increased rates of upper respiratory infections, decreased immunoglobulin levels, and injection-site reactions, underscoring the need for careful monitoring.
The trial demonstrated that telitacicept’s mechanism—targeting both BAFF and APRIL pathways—effectively suppresses B-cell overactivation, a key driver of autoimmunity in SLE. Compared with placebo, participants receiving telitacicept showed more frequent achievement of composite clinical response endpoints, including SRI-4 and BICLA measures. Despite the therapeutic benefit, safety data revealed that reductions in immunoglobulin concentrations could predispose some patients to infections, primarily mild to moderate in severity. Injection-site reactions were also noted but were generally self-limiting. The overall risk-benefit profile favored telitacicept, particularly for patients with persistent disease activity despite conventional treatment.
In conclusion, the findings suggest that telitacicept offers a promising new approach for improving disease control in patients with active SLE, though its use requires vigilance for infection risk and immune suppression. By targeting key B-cell survival factors, telitacicept represents a significant advancement in biologic therapy for lupus. The authors emphasize the importance of individualized dosing, infection surveillance, and long-term follow-up to optimize outcomes. As further studies expand on durability and safety, telitacicept may play a pivotal role in modern lupus management.
Keywords: systemic lupus erythematosus, SLE, telitacicept, BAFF, APRIL, B-cell therapy, infection risk, immunoglobulin, New England Journal of Medicine, clinical trial