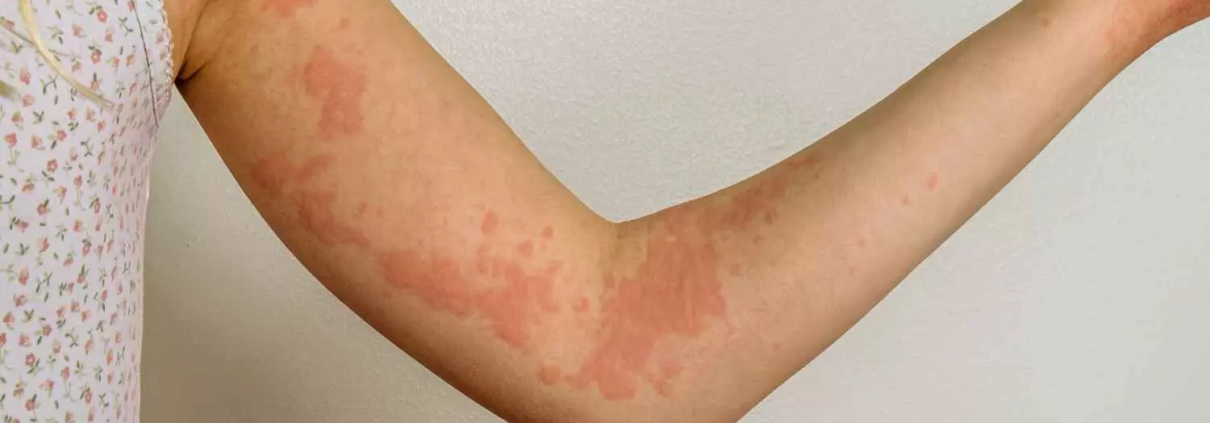
Rilzabrutinib reduces itch and hives in patients with chronic spontaneous urticaria: JAMA
A new study published in the Journal of American Medical Association found that Rilzabrutinib decreased itching and hives while preserving a positive risk-benefit profile, indicating that it might be a useful therapy for individuals with moderate to severe chronic spontaneous urticaria (CSU) that is resistant to antihistamines.
The primary cause of CSU is the activation of cutaneous mast cells via a variety of pathways. B cells and mast cells contain the protein bruton tyrosine kinase (BTK), which is essential for several immune-mediated disease processes. Thus, this research evaluated the effectiveness and risk profile of rilzabrutinib, a covalent, oral, reversible, next-generation BTK inhibitor, in the treatment of patients with CSU.
The Rilzabrutinib Efficacy and Safety in CSU (RILECSU) randomized clinical trial was a 52-week phase 2 research that included a 40-week open-label extension after a 12-week double-blind, dose-ranging, placebo-controlled period. From November 24, 2021, until April 23, 2024, the trial was held.
12 countries across Asia, Europe, North America, and South America, 51 centers recruited and randomly assigned participants. Adults with moderate to severe CSU (weekly Urticaria Activity Score [UAS7] of 16 or more; weekly Itch Severity Score [ISS7] of 8 or higher) who were not well managed with H1-antihistamine medication were enrolled in the experiment. This ranged in age from 18 to 80 and the patients were randomized 1:1:1:1 to 400 mg of rilzabrutinib, 400 mg once a day in the evening, 800 mg twice daily, 1200 mg three times daily, or a matched placebo.
160 omalizumab-naive and omalizumab-incomplete responders (mean [SD] age, 44.1 [13.4] years; 112 [70.0%] females) were randomly assigned. Only the 143 individuals who had never used omalizumab were part of the primary analysis population. ISS7 (least squares [LS] mean, −9.21 vs −5.77; difference, −3.44 [95% CI, −6.25 to −0.62]; P =.02) and UAS7 (LS mean, −16.89 vs −10.14; difference, −6.75 [95% CI, −12.23 to −1.26) showed significant decreases at week 12 when rilzabrutinib, 1200 mg/d, was compared to placebo from baseline.
Improvements were also seen in the weekly Angioedema Activity Score (AAS7) and weekly Hives Severity Score (HSS7). As early as week 1, ISS7, UAS7, HSS7, and AAS7 showed improvements. At week 12, CSU-related biomarkers, such as immunoglobulin (Ig)-G antithyroid peroxidase, soluble Mas-related G protein–coupled receptor X2, IgG anti-Fc-ε receptor 1, and interleukin-31, were lower than placebo.
Overall, along with an acceptable adverse event profile, the RILECSU randomized clinical trial findings showed that rilzabrutinib, 1200 mg/d, was effective and had a quick start of action over a 12-week period.
Reference:
Giménez-Arnau, A., Ferrucci, S., Ben-Shoshan, M., Mikol, V., Lucats, L., Sun, I., Mannent, L., & Gereige, J. (2025). Rilzabrutinib in antihistamine-refractory chronic spontaneous urticaria: The RILECSU phase 2 randomized clinical trial: The RILECSU phase 2 randomized clinical trial. JAMA Dermatology (Chicago, Ill.), 161(7), 679–687. https://doi.org/10.1001/jamadermatol.2025.0733