Oral Methotrexate Reduces Knee Osteoarthritis Pain and Stiffness Significantly in 6-Month Study
UK: A randomized,
double-blind trial demonstrates that oral methotrexate, when combined with standard treatments, showed a statistically significant reduction in knee osteoarthritis (KOA) pain, stiffness, and functional impairment at the 6-month mark. The findings were published online in the Annals of
Internal Medicine on 30 July 2024.
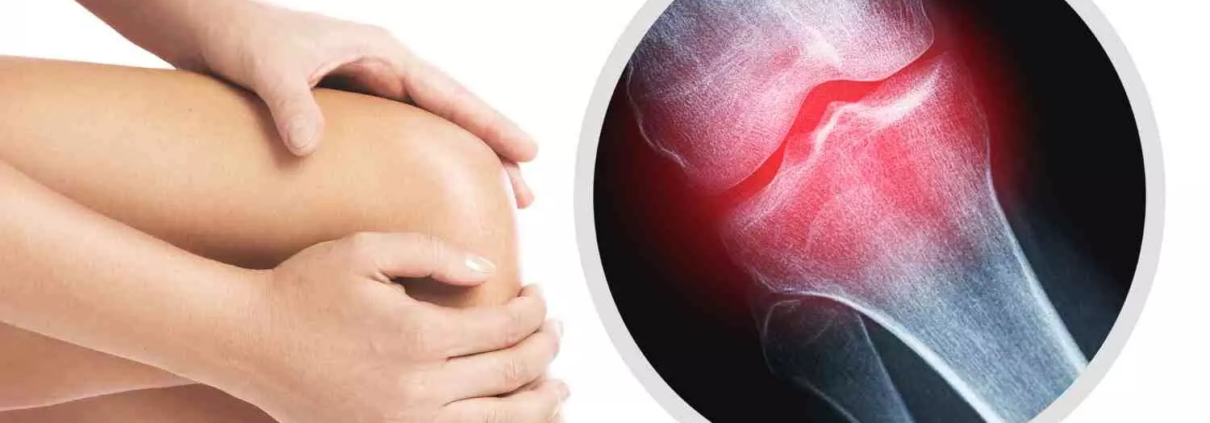
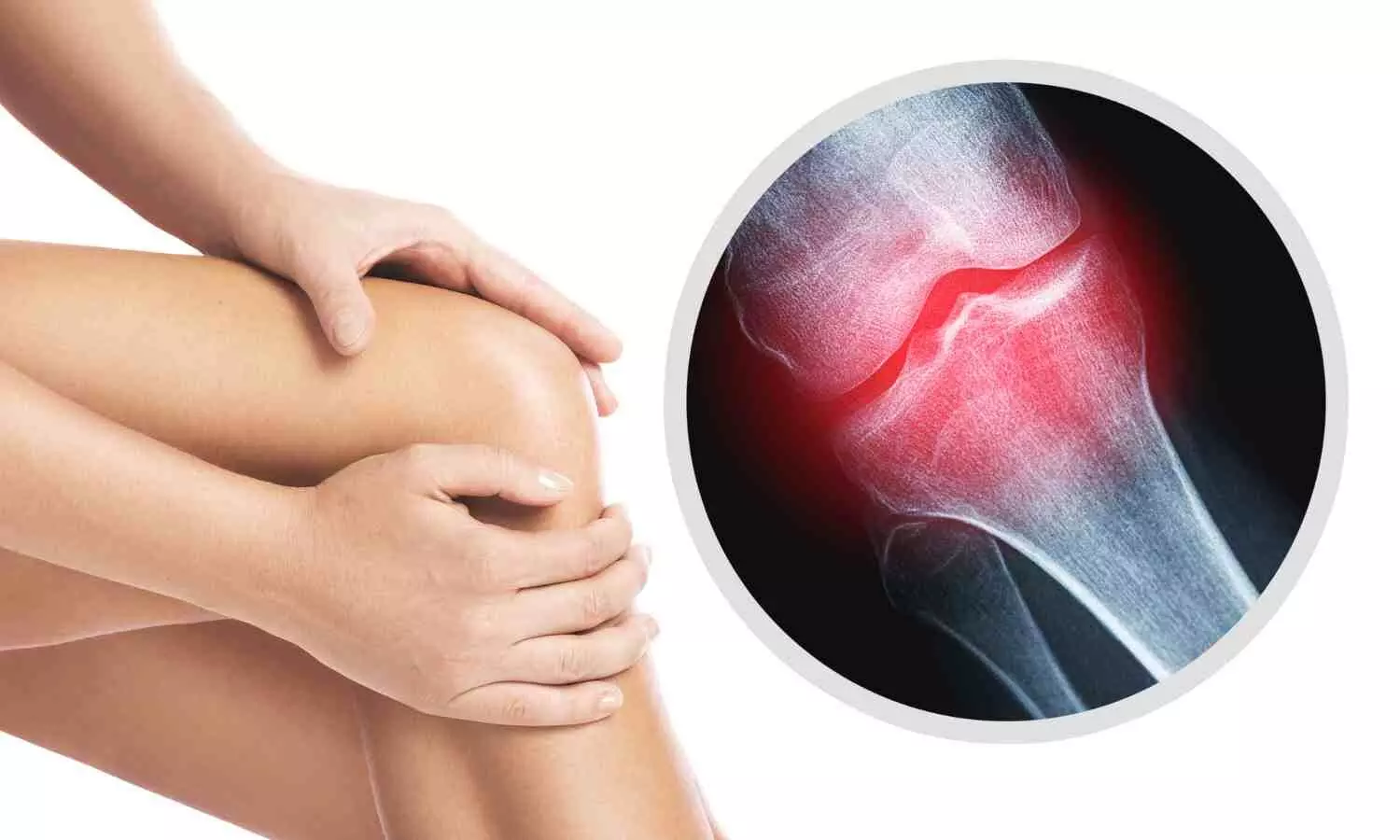
Osteoarthritis is
classified as a degenerative disease that causes subchondral sclerosis,
subchondral cysts, bone remodeling (osteophyte production), and loss of
articular cartilage. The illness is typified by joint discomfort, functional
disability, and a marked decline in life quality. Conservative (non-operative)
and surgical (operative) approaches are typically used in KOA management. Pain,
stiffness, restricted joint mobility, and muscle weakness are common KOA
symptoms that severely hinder day-to-day activities and job performance.
Considering this, Sarah R. Kingsbury, from Leeds Institute of Rheumatic and
Musculoskeletal Medicine, University of Leeds, and National Institute for
Health and Care Research (NIHR) Leeds Biomedical Research Centre, Leeds, United
Kingdom, et.al conducted a study to assess the symptomatic benefits of
methotrexate in Knee Osteoarthritis.
For this purpose, the
research team conducted a randomized, multicenter, double-blind, placebo-controlled
trial involving 207 participants from the United Kingdom. The trial was
conducted from 13 June 2014 to 13 October 2017 in the 15 secondary care
musculoskeletal clinics in the United Kingdom.
The study included 207
individuals who had radiographic KOA and knee pain (severity ≥4 out of 10) on
most days during the previous three months and were not responding well to
their existing treatment were contacted. Over 12 months, they were randomized
1:1 to receive oral methotrexate once weekly (6-week escalation: 10 to 25 mg)
or a matched placebo, while continuing with their regular analgesic regimen. Average
knee pain (numerical rating scale [NRS] 0 to 10) at six months was the main
outcome, and a 12-month follow-up was conducted to evaluate the longer-term
response. Adverse events (AEs) and outcomes related to knee stiffness and
function were included as secondary end goals.
The findings revealed
that:
- 64% of women with Kellgren-Lawrence grades 3
to 4 received methotrexate at random. At six months, 86% of respondents were
still following up. - In the methotrexate group, mean knee pain
dropped from 6.4 at baseline to 5.1 at 6 months, while in the placebo group, it
reduced from 6.8 to 6.2. - A statistically significant pain decrease of
0.79 NRS points in favor of methotrexate was observed in the primary
intention-to-treat analysis. - At six months, the McMaster University
Osteoarthritis Index stiffness and function for Western Ontario and
methotrexate showed statistically significant differences across treatment
groups. - A dose-response impact was substantiated by a
study of treatment adherence. Four separate major adverse events were noted.
“Throughout six months,
the addition of oral methotrexate to standard therapies considerably decreased
pain, stiffness, and functional impairment in patients with osteoarthritis of
the knee. Hence methotrexate is proven safe to use for the treatment of
osteoarthritis of the knee”, researchers concluded.
Reference
Kingsbury, S. R.,
Tharmanathan, P., Keding, A., Watt, F. E., Scott, D. L., Roddy, E., Birrell,
F., & Conaghan, P. G. (2024). Pain reduction with oral methotrexate in knee
osteoarthritis: A randomized, placebo-controlled clinical trial. Annals of
Internal Medicine. https://doi.org/10.7326/M24-0303