Lebrikizumab Shows Strong Efficacy and Safety in Atopic Dermatitis: Study
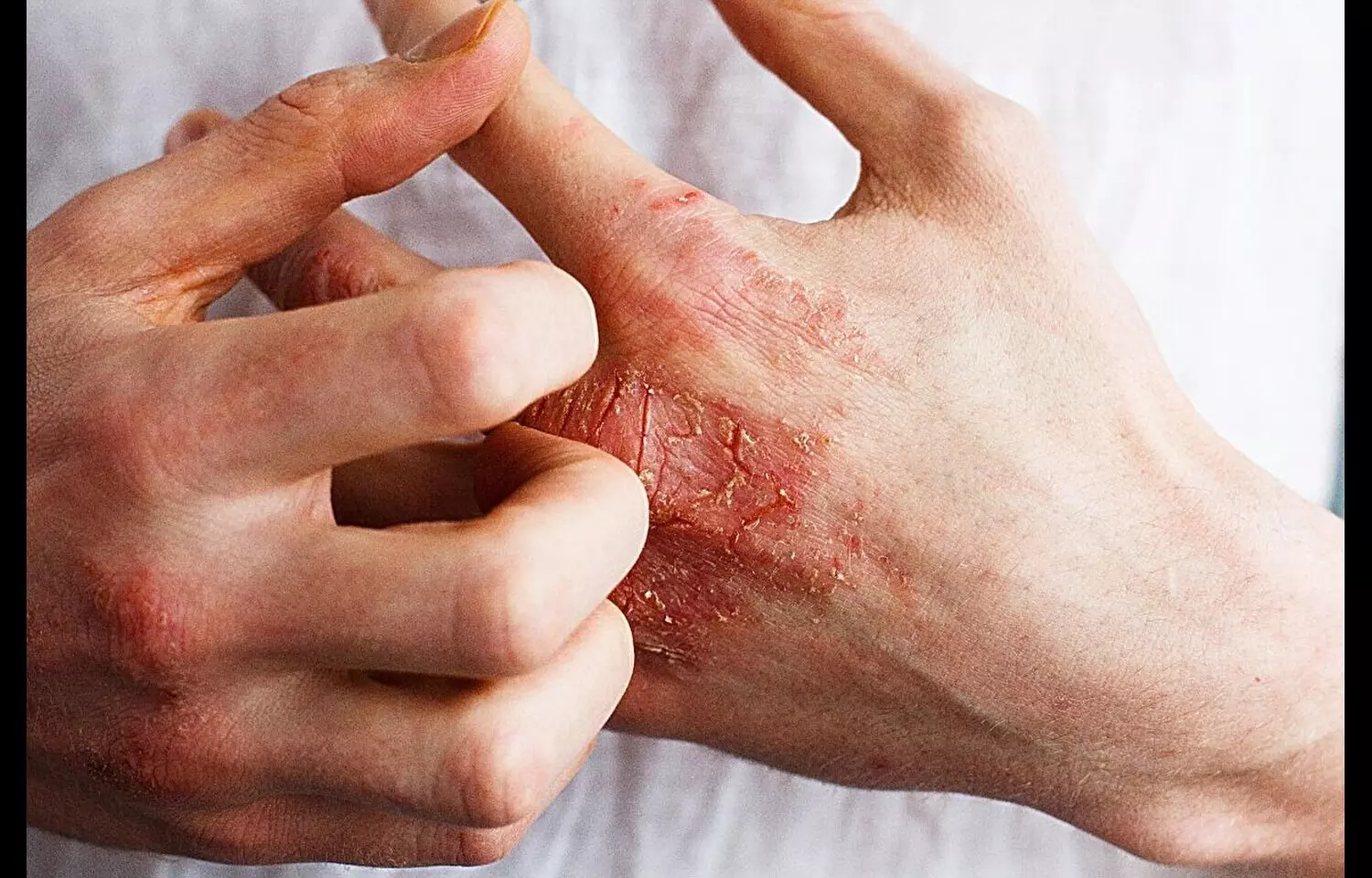
Atopic dermatitis (AD) is a chronic, relapsing inflammatory skin disease that significantly impacts quality of life and often requires systemic therapies for patients with moderate-to-severe disease. With increasing focus on targeted biologic therapies, anti–IL-13 monoclonal antibodies have emerged as a promising treatment option for patients inadequately controlled with conventional therapies.
Findings from the BIOREP registry have highlighted that lebrikizumab achieved significant improvement in atopic dermatitis, with 63.7% of patients reaching an EASI-75 response at 16 weeks. The benefit was especially pronounced in treatment-naïve patients compared to those previously exposed to biologics. The therapy also demonstrated a favorable safety profile, with sustained efficacy documented through 24 weeks. Registry data showed that lebrikizumab not only improved objective disease severity scores but also enhanced patient-reported outcomes such as pruritus reduction and quality of life measures. Rates of adverse events remained low, with conjunctivitis and injection-site reactions being the most commonly reported but generally mild. No new safety concerns were identified during the observation period. The study authors emphasized the importance of early initiation, noting that biologic-naïve patients responded more robustly than previously treated patients. This suggests that earlier use of lebrikizumab may maximize clinical benefit and minimize disease burden. In addition, the durability of response over 24 weeks supports its potential as a long-term management option in clinical practice.
As the treatment landscape for atopic dermatitis continues to expand, real-world registry data such as BIOREP provide valuable evidence on the efficacy and safety of emerging biologics beyond controlled clinical trials. The findings reinforce lebrikizumab as a strong therapeutic candidate for patients with moderate-to-severe AD who require systemic intervention.
Keywords: lebrikizumab, atopic dermatitis, EASI-75, BIOREP registry, biologic therapy, IL-13 inhibitor, treatment-naïve, real-world data, safety profile, dermatology

