FDA Approves Sapien 3 TAVR for Asymptomatic Severe Aortic Stenosis
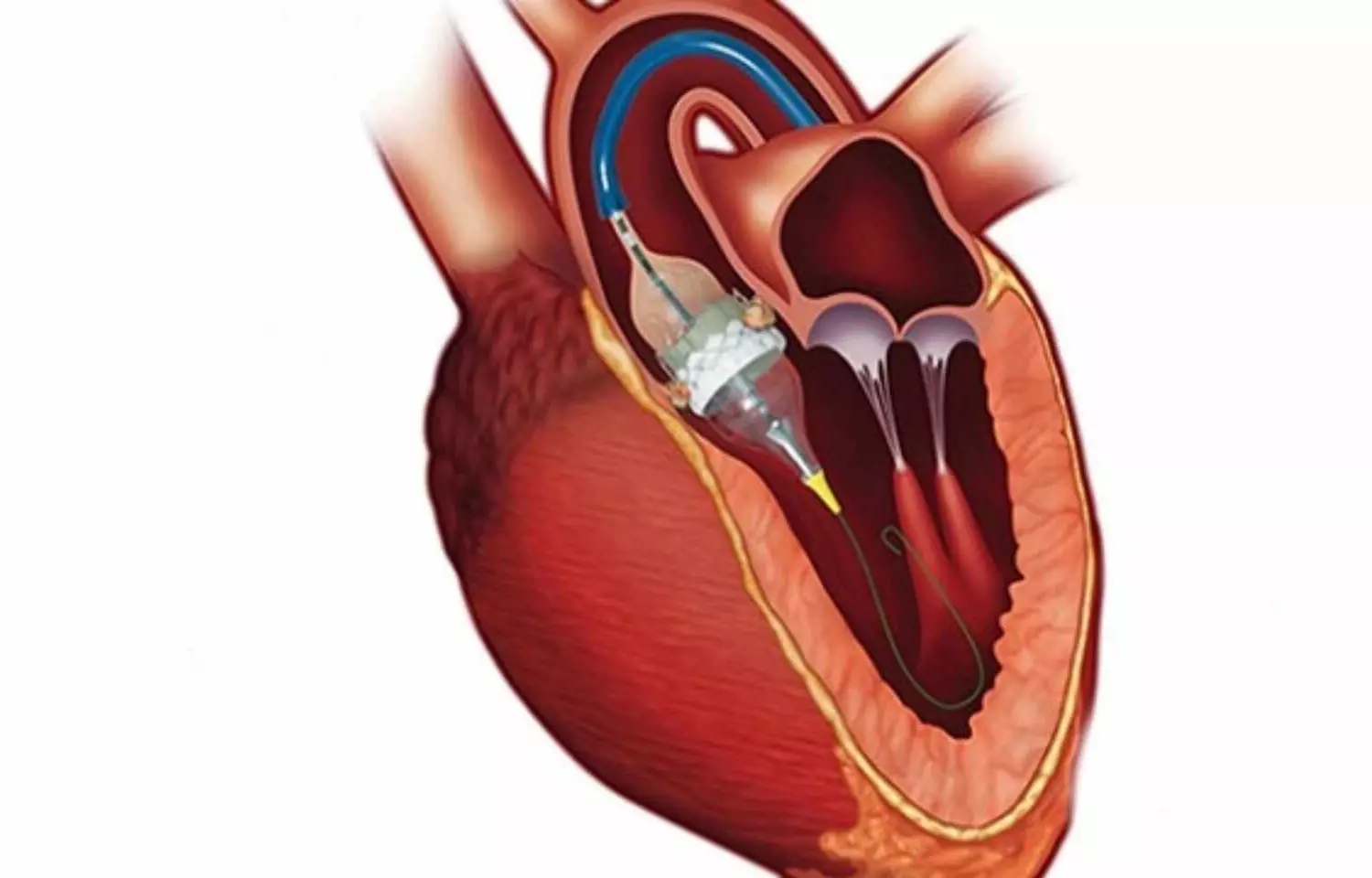
The US Food and Drugs Administration ( FDA) has approved the Sapien 3 transcatheter aortic valve replacement (TAVR) system for use in patients with severe aortic stenosis who are asymptomatic. This marks the first approval of a TAVR device for early intervention before symptoms appear, according to Edwards Lifesciences.
Approval of the SAPIEN 3 platform (SAPIEN 3, SAPIEN 3 Ultra and SAPIEN 3 Ultra RESILIA) is based on groundbreaking data from the EARLY TAVR trial, which demonstrated that asymptomatic severe AS patients randomized to Edwards TAVR experienced superior outcomes compared to guideline-recommended clinical surveillance (watchful waiting).
Without treatment, 1 in 10 patients experiencing symptoms of severe AS may die within five weeks. However, the symptoms of severe AS can be difficult to detect and may progress rapidly and unpredictably.
“There is an urgent need to change practice and TAVR guidelines for the treatment of aortic stenosis patients, which currently recommend ‘watchful waiting’ until symptoms develop,” said Philippe Genereux, M.D., director of the structural heart program at Gagnon Cardiovascular Institute, Morristown Medical Center, Morristown, New Jersey. “As we saw in the EARLY TAVR trial, patients originally designated as asymptomatic became symptomatic in sudden and unpredictable ways, underscoring the importance of early evaluation by a heart team to improve patient outcomes and benefit the healthcare system.”
The EARLY TAVR trial was the first randomized, controlled trial designed to evaluate TAVR compared to watchful waiting for patients with asymptomatic severe AS. With a median follow-up of 3.8 years, 26.8% of the 455 patients in the TAVR arm experienced death, stroke, or unplanned cardiovascular hospitalization, compared with 45.3% of the 446 patients in the clinical surveillance arm. The data were published last year in The New England Journal of Medicine (NEJM), marking the ninth NEJM publication on Edwards TAVR.
“This approval is a powerful opportunity to streamline patient care and improve the efficiency of the healthcare system,” said Larry Wood, Edwards’ corporate vice president and group president, Transcatheter Aortic Valve Replacement and Surgical. “We are proud to partner with leading physicians to advance our knowledge of this deadly disease with high quality science and optimize the treatment pathway for patients.”
Since its introduction more than two decades ago, SAPIEN has become the most studied valve platform, demonstrating unmatched clinical outcomes and solidifying its position as the leading choice for physicians and patients. More than 1 million patients have been treated with SAPIEN valves worldwide.
