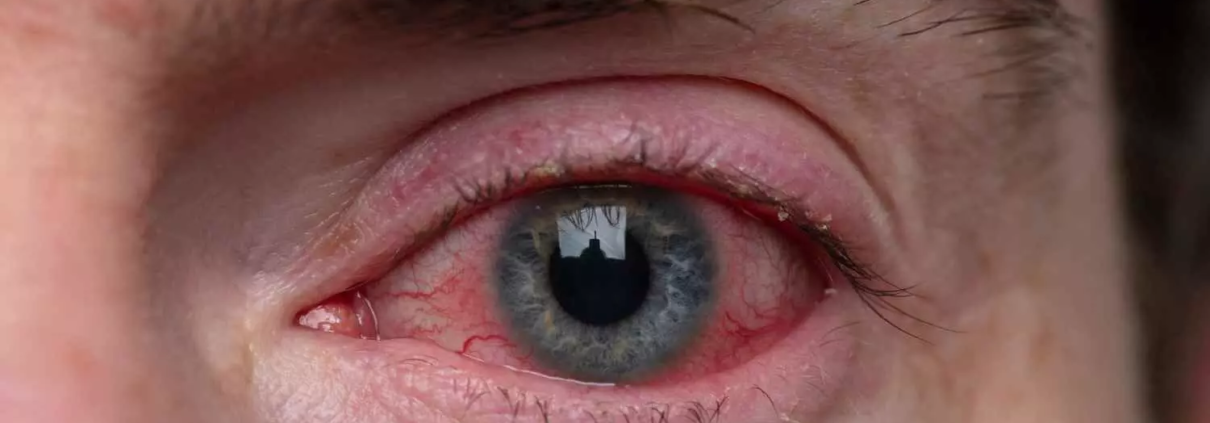
FDA Approves Prednisolone Acetate Ophthalmic Suspension for Ocular Inflammation
The FDA has approved preodnisolone acetate ophthalmic suspension, USP 1%, for treating steroid-responsive ocular inflammation. Amneal Pharmaceuticals plans to launch the product in Q3 2025.
Prednisolone acetate ophthalmic suspension, USP 1% is a sterile, topical anti-inflammatory 0agent for ophthalmic use and is indicated for treating steroid-responsive ocular inflammation.
“Our Affordable Medicines portfolio continues to grow with a strong and diverse pipeline that supports broader access to high-quality treatments across the U.S. healthcare system,” said Andy Boyer, Executive Vice President and Chief Commercial Officer, Affordable Medicines. “The approval of prednisolone acetate ophthalmic suspension-a complex product to develop and manufacture-highlights the depth of our R&D capabilities and the strength of our manufacturing and supply operations.”
The most commonly reported adverse reactions for prednisolone acetate ophthalmic suspension in clinical studies were elevation of intraocular pressure (IOP) with possible development of glaucoma and infrequent optic nerve damage, posterior subcapsular cataract formation, and delayed wound healing. For prescribing information, see package insert here.
According to IQVIA® U.S. annual sales for prednisolone acetate ophthalmic suspension for the 12 months ended April 2025 were approximately $201 million.