Dexamethasone Implant Promising as Adjunctive Therapy in Coats’ Disease: Study Finds
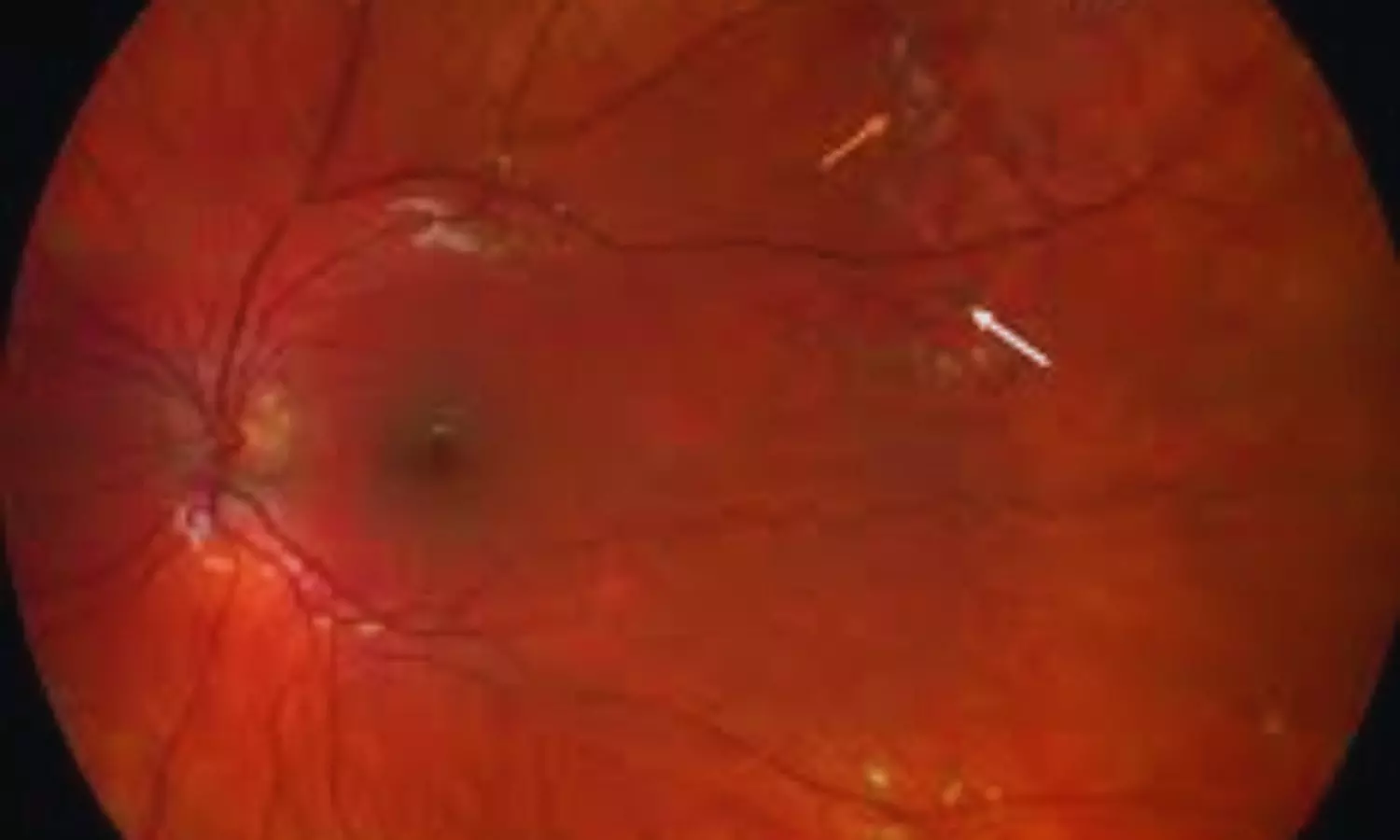
Turkey: A retrospective single-center study published in BMC Ophthalmology has highlighted the potential role of intravitreal dexamethasone implants as an adjunctive therapy in managing Coats’ disease alongside conventional ablative treatments. The research, conducted by Ozlem Ural Fatihoglu and colleagues from the Department of Ophthalmology at Dokuz Eylül University, İzmir, Turkey, assessed the efficacy and safety of this approach over a long-term follow-up period.
The study evaluated patients treated between February 2012 and January 2024 who received a dexamethasone (Dex) implant in addition to standard ablative therapy, with a follow-up duration exceeding 12 months. Out of 15 enrolled patients, the analysis included 11 patients. The cohort primarily consisted of males (90.9%) with unilateral disease, while the only female participant presented with bilateral involvement. The mean age at diagnosis was 14.8 years, spanning a wide age range from 2 to 60 years, highlighting the diverse presentation of the disease.
The following were the notable findings of the study:
- At baseline, 58.3% of the eyes were classified as stage 3a1, 25% as stage 3a2, and 16.7% as stage 2b.
- Over an average follow-up of nearly 66 months, visual acuity remained stable in 50% of the treated eyes.
- One-third of the eyes showed measurable improvement of at least one Snellen line.
- Two eyes demonstrated a two-line gain, while the other two eyes achieved a single-line gain in visual acuity.
- Vision deteriorated in both eyes during the study period.
- Transient intraocular pressure (IOP) elevation was observed in 66.7% of treated eyes.
- Cataract progression occurred in 58.3% of eyes, with one-third requiring cataract surgery.
- One eye developed a secondary vasoproliferative tumor, necessitating CyberKnife therapy and vitrectomy.
- No severe implant-related complications were reported, indicating a favorable safety profile for the treatment.
The findings suggest that intravitreal dexamethasone implants may serve as a useful adjunctive option, particularly in pediatric patients, to reduce the need for repeated general anesthesia and in adults with extensive macular exudation, where conventional ablative therapy alone may be insufficient. While the outcomes are promising, the authors acknowledge key limitations, including the retrospective design, small sample size, absence of a control group, and the study’s setting in a tertiary referral center, which may have resulted in a higher proportion of advanced cases.
Despite these limitations, the research highlights the value of dexamethasone implants in achieving anatomical and functional stabilization in Coats’ disease. With half of the treated eyes maintaining stable vision and one-third showing improvement, the study adds evidence supporting the integration of steroid implants into multimodal treatment strategies,” the authors wrote.
They recommend further studies to validate these findings and better define patient subgroups that may benefit most from this adjunctive approach.
Reference:
Ural Fatihoglu, O., Ayhan, Z., Ozturk, T. et al. Place of dexamethasone implant as an adjunctive treatment in Coats’ disease: a retrospective single-center study. BMC Ophthalmol 25, 435 (2025). https://doi.org/10.1186/s12886-025-04279-2



